Disclaimers: I own Veeva shares; this is not investment advice
Veeva is the global leader in cloud software for the life science industry, serving ~1,500 customers, including ~47 of the top 50 global biopharmaceutical companies.
Since its IPO in 2013, Veeva has grown its revenue more than 13-fold (a ~26% CAGR) while maintaining best-in-class operating margins (~40% non-GAAP over the last five years).
Yet despite this level of profitability, Veeva has spent the past 4+ years investing heavily in a range of new applications. These products are reaching maturity and beginning to take real share in markets where Veeva is generally out-innovating legacy providers. These markets are also large — many of them are ~$1bn opportunities.
With its tentacles now spanning virtually the entire commercial and R&D functions of its customers, Veeva is exceptionally well-positioned to bring AI to the industry. After some initial skepticism on the technology, Veeva’s CEO stated just last quarter that through AI he thought Veeva could increase the efficiency of the entire life science industry by ~15% over ~5 years. Across a $2 trillion industry, that would represent a staggering ~$300bn in efficiencies! Veeva’s AI offerings are slated to begin rolling out in earnest around the end of the year.
While Veeva will undoubtedly grow from here, is the future so bright that it justifies Veeva’s current ~15x revenue multiple? I’ll examine below.
Business Overview
Veeva was founded in 2007 by Peter Gassner and Matt Wallach. Its original product was a life science-focused CRM, built on top of the Salesforce platform.
The company chose life sciences as its initial area of focus because “horizontal” CRM solutions simply wouldn’t suffice for the industry’s complex and regulation-heavy set of requirements. Furthermore, at the time, Veeva benefitted from (1) there being no true cloud (i.e. multi-tenant) solution for the industry and (2) the fact that the industry’s primary incumbent — Siebel — had a solution so byzantine that certain clients reportedly felt it necessary to spend $5-10m on projects just to reduce its complexity.
Veeva won its first contract with Pfizer — on a niche product with just 6 sales reps. For the next ~12-15 months, Veeva focused the entire company solely on Pfizer’s success as a client. Veeva would graduate to a 23-rep product, then a 35-rep product, before winning an RFP for Pfizer’s entire US sales force. After cementing its position with Pfizer, Veeva continued to focus solely on CRM — first within just the US and later globally.
In 2012, Veeva began introducing products for the R&D (or “drug development”) function of its life science customers — a function basically entirely separate from CRM/commercial.
Over a decade of cross-selling ensued and continues to this day (in fact Veeva is still in the earlier innings of this). As it turned out, this was a very large opportunity as well as a landscape littered with point solutions.123 Veeva has replaced up to 200 different vendors for certain customers over time, generally charging less (and offering a better product) while itself generating super normal profitability.
Looking back, it was the perfect strategy at the perfect time with the perfect team…
Using just $7m of VC capital (and spending only $3m of that), Veeva today sports a market cap of ~$48bn on ~$3bn of revenue. It sells over 50 different applications across the commercial and R&D functions, with revenue split roughly 50/50 between the two areas.
Key Investment Merits
Extremely Sticky
Veeva is maybe the preeminent example of vertical SaaS stickiness:
The software is built from the ground up for the life science industry. In life sciences, regulatory forces are onerous and change by the geo (from country to country and even state to state).4 Things like “pharmacovigilance” must be tracked meticulously5 and applications that seem simple on the surface, like Events Management, are muddied by industry-specific compliance requirements.6 Veeva has a deep, deep understanding of the industry and offers products that are built from the ground-up to address these requirements.
The software is mission critical. Pharma companies can’t operate without Veeva’s solutions. In some cases (i.e. the Safety suite), if Veeva were to fail, a customer would have to immediately stop all commercial activities.
Veeva’s offerings are predominantly “system of record” solutions. Veeva acts as the primary, authoritative database for much of the data it touches. This increases switching costs in a major way.
The software consists of integrated suites of solutions. These suites are designed to talk to each other seamlessly and remove very material integration headaches for Veeva’s customers. Customers are thus incentivized to “standardize” on Veeva, or adopt Veeva for every application in a suite.
Veeva has a highly trusted brand. Veeva has become the “no one gets fired for buying IBM” brand. Except it’s really that on steroids — many Veeva offerings are in areas of ultra-high conservativism for its customers (clinical trials, safety, quality). You simply cannot mess up one of these applications.7
For all these reasons, a competing product doesn’t stand much of a chance — and indeed, Veeva really hasn’t seen any credible new entrants since its founding ~20 years ago. A competing solution would need to be perhaps 2x better to have any real shot at success. And there’s just no way to be 2x better at this point over any reasonable period (expect perhaps through through a paradigm shift like AI — I’ll address that later).
Product Excellence
From its founding, Veeva’s greatest priority has been product excellence. From its 2014 investor day:
“So if you look at Veeva and how we're founded, we were founded by some super product people. I'm a product person in my bones. And we have people that know how to really develop product, design product, market product, sell product, service product. Product, product, product. And we decided, and we wrote this down very early in the company, probably within the first 3 months, we're going to have product excellence.”
Veeva’s approach to product is that every application it introduces should win on a standalone basis, or be “best of breed.” The fact that an application integrates neatly with the suite around it should simply be an added bonus — not the reason for winning. From its 2015 investor day:
“When we say it's integrated and best-of-breed, best-of-breed means if they were going to go do an RFP for just e-mail, if we have an e-mail product, they should be choosing ours. We're going to invest until it is the very best product in the market, and the fact that it's beautifully, perfectly integrated with the rest of the Commercial Cloud, it's kind of gravy.”
Getting there isn’t easy. According to management, it requires great product people working intelligently for 4+ years. It requires making the right product decisions over and over again, day in and day out. And while it’s a subjective metric, I think its fair to say Veeva has achieved this (or will achieve it) across the majority of its offerings.
Product excellence makes everything easier:
It makes sales easier. Sales come much easier when you have a reputation for great products. Even more so when you are selling to a customer where you’ve already sold and delivered on great products.
It makes professional services easier. Veeva believes its professional services personnel enjoy their jobs more/have longer tenures because they are working with customers who generally like and appreciate Veeva’s products.
It improves morale throughout company. If you are constantly dealing with customers that like & appreciate you, you will have a lot better morale than if you are dealing with customers that don’t.
Product people enjoy working there. Product people at Veeva are building the industry’s best products for the industry’s leading customers.
Gassner, a platform engineer by trade, still reportedly sits amongst Veeva’s platform engineering team.
Customer Goodwill
Veeva prides itself on its customer friendly behavior.
Veeva has a goal of being the “leader and liked.”8 They want to be essential to the industry, but also appreciated by the industry. From a Jan-23 JPM conference:
“We want to be essential to each company in the industry and appreciated by each company in the industry. That's a super high bar because when you become essential, truly essential, you tend to not be appreciated because there's some resentment towards that. And you also get — you get arrogant and lackadaisical when you're essential. But that's our bar. We want to be essential to the industry and appreciated by the industry.”
Some examples of their customer-friendly behavior:
Lack of price increases. Veeva didn’t increase subscription pricing on existing customers from 2007 through — wait for it — 2023. Yes, that’s right — zero price increases over 16 years despite a clear ability to do so (and a competitive set that was supposedly raising prices by ~5-7% annually). When Veeva was ultimately somewhat forced to as a result of COVID-era inflation, they pegged increases to the lesser of CPI or 4% annually.
PBC. Veeva converted to a public benefit corporation (PBC) in 2020. This means that Veeva now has a legal obligation to balance the interests of customers, employees and shareholders (vs. just prioritizing shareholders). This was always how Veeva strived to operate in reality, but the PBC conversion made it a legal duty at the Board level. Management believes this will help hardcode such behavior into the company’s DNA for the long-term — beyond the tenures of Gassner, Wallach & co.
Understaffing sales. Management believes in purposefully understaffing its sales team because it leads to better sales behavior.910 They don’t want reps using aggressive tactics to make a quarter or go for the last dollar; they want to incentivize long-term relationship building. Maintaining a high number of opportunities per rep and achievable quotas helps accomplish this.
Annual contracts. When Veeva launched, its contracts were annual which was a departure from the multi-year industry standard. Primarily Veeva did this because it didn’t want to discount its offering, it should be noted. But Veeva also did it to establish a mindset of having to win a customer’s business every year — to ward off laziness.
While this type of behavior sacrifices some near-term profits, it’s great long-term stewardship. They do right by their customers. They deliver great products. They are okay perhaps being a little underpriced relative to the value they provide. They don’t go for the last dollar. This all results in happy customers. Happy customers buy more products and happy customers don’t churn. From its 2014 investor day:
“The last one is customer success. For those of you who have spent a lot of time with us, you know that this is not just lip service. This is what drives our company, but it is also what may keep other companies out. Because if you're going to go start a software company, you're not going to take on a company that has a big installed base of customers that are happy. You don't do that.
Enterprise software is hard. You don't want to rip that stuff out. So you have to be unhappy for a couple of years before you really want to throw out an enterprise software system. So the success that we have, our customers want us to do more, like the call that Peter got, the calls that we get every week. ‘Hey, are you going to go into this? We're going to do a project. I really don't want to buy it from that niche point vendor, would you please do it and we'll just add it to the Veeva landscape.’
So it's hard to compete against an enterprise software company with happy customers. I would never start a software company to go compete against SAP in life sciences. They're well respected, the people are humble, they're still working hard even though they've sort of sold out the patch. You wouldn't do that because companies are happy with SAP.
By the same token, they're happy with Veeva. And so we're not so much of a target. And we actually haven't seen any companies start and compete with us with multi-tenant cloud applications in any of the markets that we compete with.”
Host of Emerging Products
Veeva has developed a host of new applications over the last 4+ years that have really just begun to mature and generate revenue.
The chart below from Veeva’s latest investor day shows certain of its large product categories by product maturity and market share. As you can see, most are still in the bottom-left quadrant.
These products are in markets that are generally large and underserved by smaller, legacy competitors.
I believe this has Veeva well-positioned for its next leg of growth — I discuss extensively later on.
Great Team
Peter Gassner has served as Veeva’s CEO since the company’s founding. Gassner and team (the senior team has an average tenure of ~10 years) have a distinguished track record.
Built a ~$48bn market cap company on just $7m of VC capital
Grown revenue by 13x since its IPO in 2013, a CAGR of 26% over 11 years
Achieved a GAAP operating margin in the mid-20%s and a non-GAAP operating margin in the low-40%s, both best-in-class vs. software peers
All while setting the company up for a strong next leg of growth with a host of emerging and promising products
While getting up there in age, I’d expect Gassner to lead the company for at least the next 5 years.
Tremendous Financial Model
It’s got everything you could ask for:
High gross margins
High operating margins
Minimal capital requirements
High revenue per employee
Substantial consistent excess cash flow
High ROE
100%+ net dollar retention
Exceptional CLTV/CAC metrics
“Rule of 40” vs. peers:
CAC payback vs peers11:
About as Non-Cyclical as You Can Get
Veeva supplies mission critical systems to a non-cyclical end market. Patients need their medicine no matter the macro environment.12 ~83% of Veeva’s revenue is subscription-based.
Well Positioned on AI
In short, Veeva seems extremely well positioned for AI. I think for Veeva, AI will be a “sustaining” technology and not a “disruptive” one. And I think Veeva will benefit from a larger TAM, even more compelling products and/or internal efficiency gains. I examine later in greater detail.
Product Overview
Veeva’s product portfolio extends across the “commercial” (i.e. sales & marketing) and “drug development” (i.e. clinical trials through manufacturing and safety monitoring) functions of its life science customers.
This is what Veeva is going for, essentially (from its 2017 investor day):
SAP can handle the ERP; Veeva will take everything else…13
Today Veeva has 50+ applications that fall into 5 main product categories:
Commercial Systems (e.g. CRM, PromoMats, MedComms, Crossix, Compass)
Drug Development Systems:
Clinical Operations (e.g. eTMF, CTMS, RTSM)
Clinical Data Management (e.g. EDC, CDB)
Regulatory & Safety (e.g. Safety, Submissions)
Quality (e.g. QualityDocs, QMS, LIMS)
As you can see above, Veeva’s solutions look like an “alphabet soup” and are not easy to keep straight upon first pass (or second or third…).
Below I’ll try to shed some light on each area.
Commercial Systems
Veeva’s first product was a life science specific CRM developed on top of the Salesforce platform and introduced in ~2007. Today Veeva dominates this niche, with ~80% market share of all pharma sales reps globally.
Over time Veeva developed a suite of complementary add-on applications, which altogether Veeva refers to as its “Commercial Cloud.”
Below is a look at how some of its core commercial products have penetrated the world’s top 20 pharma companies over time.
Note: the top 20 pharma charts that follow are based on “unofficial” data. Veeva does not formally report on these metrics, say every quarter. Rather, they provide intermittent disclosures, such as after a new top 20 win, in earnings releases or on investor calls. So while I think they’re useful, they won’t be fully accurate (and some could be materially off).
Veeva’s count of top 20 pharma customers is an important metric because the industry is disproportionately top-heavy — the top 20 represent approximately half of all industry revenue.
Note the CRM line and the PromoMats line are overlapping — they both count 19 of the top 20 pharma companies as customers, which was first disclosed by the company at its 2017 investor day. Veeva’s top 20 CRM customer count will likely tick down a bit as a result of a CRM platform shift that is underway — I discuss this later. Also the Compass/Crossix wins solely represent brand wins (of at least one) within a top 20 pharma customer (and are far from winning these customers’ entire portfolio of brands).
As we can see above, Veeva has dominated CRM/PromoMats for many years, and is only just beginning to scratch the surface in the Crossix/Compass markets.
Below is some detail on the commercial suite beyond the CRM:
PromoMats (announced 2011): content management application supporting the full lifecycle of promotional content. It enables content creation, review and approval (a critical function in life sciences), digital asset management (DAM), etc. PromoMats is connected with CRM for automatic distribution of promotional content via applications like CLM (Closed Loop Marketing) and Approved Email
MedComms (announced 2011): content management application supporting the specific needs of Medical Affairs (a function within the pharma industry that focuses on communicating scientific and clinical information about a company’s products to HCPs and the public). It enables content creation, review and approval, storage, management, and distribution.
Approved Email (announced 2013): compliance management for email communications with HCPs (healthcare providers)
Events Management (announced 2015): end-to-end compliant event management
Align (announced 2015): sales rep territory management
Crossix (acquired 2019): (1) marketing spend measurement & optimization and (2) programmatic advertising. Crossix is supposedly the clear market leader in measurement and earlier in programmatic advertising.14
Service Center (announced 2023): application for inside sales, contact center, and hybrid reps that enables inbound and outbound engagement across channels [still in early development]
Campaign Manager (announced 2024): application for marketers to create and execute HCP campaigns [still in early development]
Veeva also has a set of data products that tie mostly into the commercial suite, designed to optimize and enhance the S&M function. Veeva calls this its “Data Cloud.”
OpenData: global reference data of healthcare professionals (HCPs) and healthcare organizations (HCOs). It contains HCP names, addresses, contact information, specialty, compliance data (license information and industry identifiers), and affiliations (and is available in 100+ countries). I believe the vast majority of Veeva’s CRM customers also subscribe to OpenData.15
Link: deeper data on key accounts/key opinion leaders, etc. A “life science-specific LinkedIn on steriods”16
Pulse: benchmarking data based on anonymized commercial activity across Veeva’s entire CRM customer base. Veeva is introducing the Pulse product within R&D as well (for its applications that help manage clinical trials)
Compass:
Compass Patient: Compass Patient is anonymous patient longitudinal data, including dispensed prescriptions and procedures and diagnoses for the U.S. market
Compass Prescriber: Compass Prescriber is projected prescriptions and procedures at the HCP, HCO and Zip level for retail and non-retail products. It covers more than 4,000 brands for the U.S. market
Compass National: Compass National is projected prescriptions and procedures at the state and national level for retail and non-retail products. It also covers more than 4,000 brands for the U.S. market.
Drug Development Systems
Starting in 2012, Veeva began introducing software for the drug development function. This function is basically entirely separate from commercial, so this was not an “add-on” product to its existing suite, but more of an exercise in “starting from scratch.”
Today, Veeva’s “development” revenue represents ~50% of its total revenue, and is expected to represent about 2/3rds of total revenue by 2030.
Clinical Operations
Veeva’s clinical operations suite helps life science companies build and execute clinical trials.
As you can see below, Veeva has steadily built a dominant position in the market. Veeva counts 19 eTMF customers and 17 CTMS customers in the top 20 as of Q1’FY26 (Veeva expects the final top 20 to select Veeva eTMF this year, per the Jun-25 William Blair conference).
Veeva’s first product in the suite was eTMF, introduced in 2012. eTMF, or electronic trial master file, is a content management platform for clinical trials. It houses all documents related to clinical trials, and provides full enterprise content management capabilities for upload, version control, QC/approval, and real-time co-authoring with Microsoft Office for study documents.
As of Q1’FY26, 19 of the top 20 pharmas use it (450+ customers total), as do at least 4 of the top 6 CROs. The success of eTMF formed an important beachhead within clinical ops.
Veeva followed up eTMF with CTMS (Clinical Trial Management System), introduced in 2017. CTMS acts as the hub for clinical trial monitoring and management (for both insourced and outsourced trials). CTMS was designed to work seamlessly with eTMF, and together they form the core set of applications needed for clinical ops.
Veeva’s broader clinical ops suite:
Study Startup (announced 2015): manages the start-up activities of a clinical trial, including feasibility, qualification, and activation of research sites.
Site Connect (announced 2020): allows sponsors and research sites to collaborate on a trial by automating the flow of information to and from sites during start-up, execution, and closeout
Payments (announced 2020): manages reimbursements to research sites and tracks study budgets
RTSM (Randomization and Trial Supply Management; acquired in 2021): used by sponsors, CROs, and sites on clinical trials to randomize subjects and manage trial supplies
Study Training (announced 2022): manages GCP and study-specific training for research sites, CROs, and sponsor personnel. It provides document, video, and SCORM/AICC training, in addition to quizzes and classroom capabilities based on curricula and training requirements
Disclosures (announced 2023): manages the sharing of study registrations and results disclosures with registries
OpenData Clinical (announced 2023): provides compliant reference data on global investigators and research sites
Clinical Data Management
Veeva’s clinical data management suite helps life science customers collect and process the data generated by a clinical trial.
This is an area of very high conservatism for life science customers, who are quite reluctant to move off of systems that work “well enough”. Yet as we can see below, after ~6 years of development, Veeva has seen a nice uptick in customers over the last few years.
The EDC (Electronic Data Capture) application was the first product in the data management suite. Introduced in 2016, it was designed to tie into Veeva’s eTMF product. The EDC provides an end-to-end environment to collect, review, and process trial data about patients. The EDC served as a beachhead within the clinical data management space — similar to eTMF in clinical ops.
Veeva followed up EDC with CDB (Clinical Database) in 2018. The CDB aggregates, cleans, and transforms clinical data from all sources (including Veeva’s EDC, third-party EDCs, labs/imaging, ePRO, etc.). This creates a unified and harmonized dataset for a comprehensive view of trial data. It's an environment where the customer can clean and format their data so that it ultimately can be submitted to a health authority.17
Veeva’s eCOA (electronic Clinical Outcome Assessment) solution is the latest addition to the suite (announced in 2022). The eCOA captures questionnaire responses directly from clinical trial patients (ePRO), clinicians (eClinRO) or patient caregivers (eObsRO) using an app or webpage. Sponsors manage the eCOAs through their own interface, and a central library allows them to reuse eCOAs across studies, where appropriate. Sites have a simple access point to manage their participants and can review eCOA data and adherence.
Regulatory & Safety
After a clinical trial wraps, a pharma company or trial “sponsor” must build a regulatory package, submit it to the health authorities and then continue to demonstrate that its drug is safe and efficacious post trial. Veeva’s Regulatory and Safety suites support these functions.
Similar to CDMS, Veeva has been steadily gaining share over time in its RIM (Regulatory Information Management) suite. In Safety, another area of extreme conservatism for life sciences customers, Veeva, just in Q4’FY25, doubled its number of top 20 customers from two to four.
The RIM suite started with Submissions which was announced in 2013. Like eTMF, this is a content management application. It is used to plan, author, review, and approve regulatory submissions. Veeva expanded the suite with:
Submissions Archive (announced 2015): secure repository of applications submitted to health authorities
Submissions Publishing (announced 2017): generates electronic submissions for global health authorities
Veeva Registrations (announced 2015): allows sponsors to plan, track, and report on global product registrations as well as handle health authority correspondence and commitments
Once drugs have been licensed for use, sponsors must continue to monitor their effects, especially in order to identify and evaluate previously unreported adverse reactions. This is a practice known as “pharmacovigilance.”
Veeva’s Safety applications operate as a unified pharmacovigilance system. Veeva Safety (announced 2019) is a individual case safety report (ICSR) management system that manages the intake, processing, and submission of adverse events for clinical and post-marketed products (also called Adverse Event Management). Other applications in the Safety suite include:
SafetyDocs (announced 2019): content management for safety-related content and processes
Signal (announced 2020): tools for signal detection from various data sources, utilizing advanced statistical methods to identify potential safety issues
Safety Workbench (announced 2023): application designed for complex reporting and data analysis on large volumes of data within the safety suite
Quality
The Quality suite relates to the drug manufacturing process. Once a drug is approved, a sponsor must ensure its manufacturing processes meet certain quality standards.18
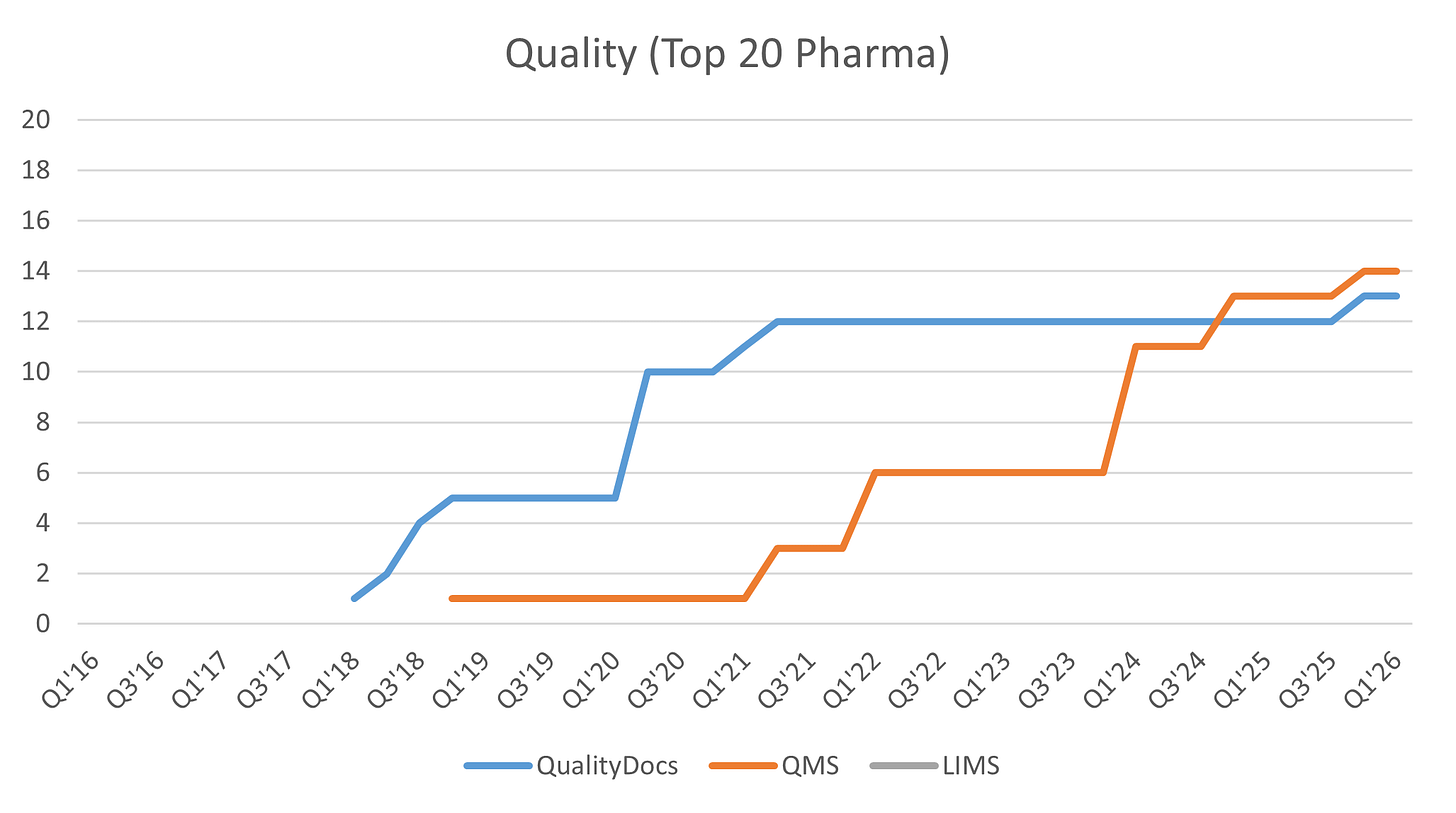
Veeva, again, has generated strong momentum in the suite, with QualityDocs and more recently QMS winning over 50% of the top 20 pharmas as customers. The LIMS application, announced in 2021, I believe has yet to win a top 20 pharma customer.
QualityDocs was Veeva’s first foray into the space, announced in 2013. QualityDocs is the industry-leading GxP quality content management application (for standard operating procedures, quality agreements, batch-related documentation, etc.), with 13 of the top 20 pharmas using the product as of Q1’FY26.
Following QualityDocs came QMS (Quality Management System) in 2016. QMS is a system designed to manage life sciences-specific quality processes. It provides best practices for handling complaints, deviations, audits, and change control. It also allows external partners to access the system to collaborate on investigations, audit findings, and corrective actions.
LIMS (announced 2021; Laboratory Information Management System) is another key product in the suite, allowing customers to manage laboratory operations more efficiently and compliantly.19
Additional products in the Safety suite include:
Training (announced 2018): a learning management system (LMS) designed for GxP compliance
Validation Management (announced 2021): streamlines commissioning, qualification, and validation activities across computerized systems, facilities, utilities, equipment, and processes
LearnGxP (acquired in 2021): an accredited training library that can be deployed with Veeva Training or other learning management system
Batch Release (announced 2023): automates aggregation, reviews, and traceability of batch-related data and content to enable faster, more confident GMP release and market-ship decisions
Next ~5 Yr Opportunity
My TL;DR view regarding the next five years is that I believe Veeva will gain significant share across the following large product categories: EDC/CDB, eCOA, RTSM, Safety, LIMS, QMS and CTMS. I believe these gains will power Veeva’s revenue from ~$3bn to ~$6bn by 2030.
It’s not an easily digestible bull case, but perhaps therein lies a bit of opportunity for investors. It’s a broad-based opportunity for Veeva across a number of areas that are (1) not easy to necessarily understand, (2) not the easiest to size from a TAM perspective and (3) subject to varying degrees of competition.
Management’s 2030 Revenue Target
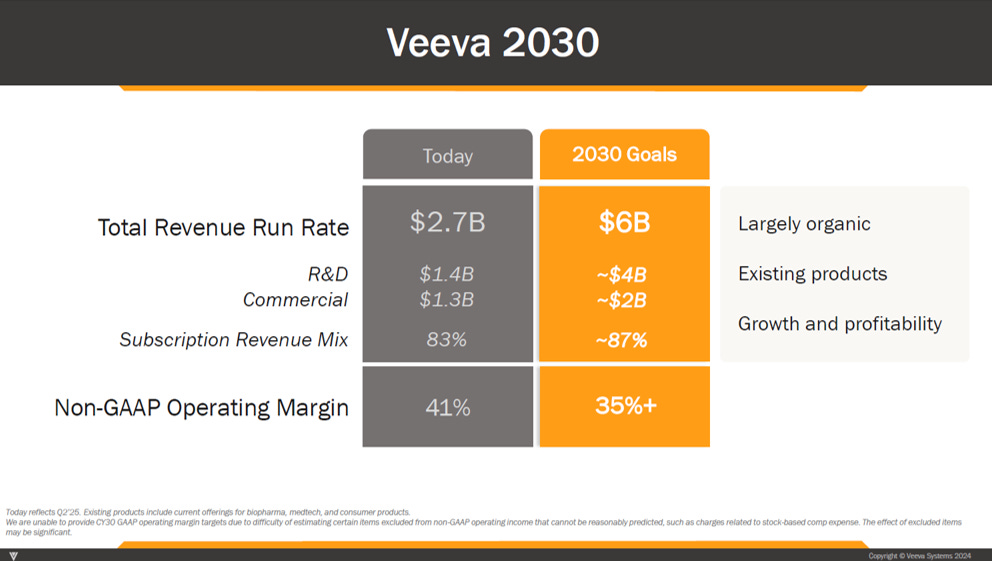
But before examining, we should note that we actually have a management target for 2030 revenue (they provided one at their 2024 investor day).
Management believes Veeva will get to a $6bn revenue run rate by calendar year 2030 (~$4bn from R&D and ~$2bn from Commercial).
And notably, management is batting 1000% when it comes to hitting long-range financial targets. They’ve previously laid out 2 long-range financial targets — $1bn in run rate revenue by 2020 (set in 2015) and $3bn in run rate revenue by 2025 (set in 2019).
Veeva achieved both targets about a year early.20
TAM Discussion
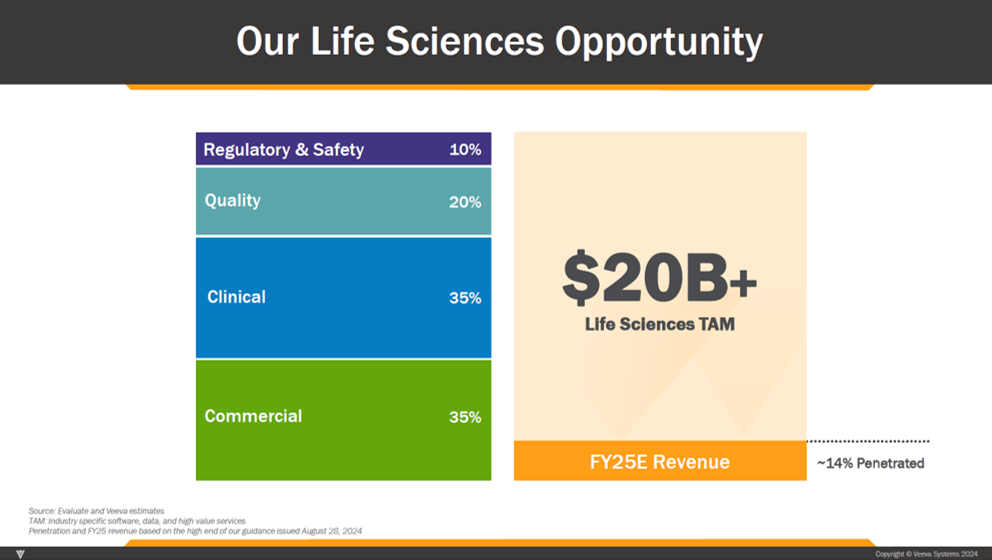
Handicapping growth by product area starts with a look at TAM by product area.
Veeva management outlines a TAM of $20bn across its current set of application areas. Within that, Commercial and Clinical (Ops + Data) represent TAMs of approximately $7bn each, Quality a TAM of $4bn and Regulatory & Safety a TAM of $2bn.
While Veeva discloses TAM at a high level for each of its product areas, it does not at the application level. So we are left to make educated guesses here based on management commentary.
In terms of Veeva’s market share by application, management kindly broke this out for us in very nice detail at its latest investor day:
Here is the commentary that came with the chart above, which summarizes some of management’s thoughts regarding its best medium-term opportunities:
“Zooming back out, this is now a broader view of Veeva's major product opportunities across the business. Five of our products are clear market leaders today, CRM, PromoMats, eTMF, Submissions and QualityDocs. Each of these were introduced more than 10 years ago, and they're some of our most mature products. Together, they account for more than half of our subscription revenue today, and they still have some room to grow. After those, we have a lot of products that are in or entering the reference selling stage, CTMS, QMS, Crossix, Link, EDC, Safety and Site Connect to name a few.
These products are the key drivers of the 2030 plan. And then there continues to be a strong pipeline of newer products that are closer to the early adopter phase, including Compass, eCOA, RTSM and LIMS.
These products will continue to contribute through 2030, but more of their contribution will likely be after 2030.”
More color21
Below we’ll try to piece together an understanding of which products are most likely to drive incremental revenue and by how much over the coming ~5 years.
EDC / CDB
Other than CTMS/QMS (more on those later), EDC/CDB is perhaps the easiest area of Veeva’s growth to handicap.
As you can see from the top 20 pharma chart, Veeva has already increased its top 20 customer count from ~2 as of Q2’FY23 to ~9 as of Q1’FY26. With 9 out of 20, by my math you are already at around a ~50% share. Yet as you can see from the market share chart above, Veeva’s EDC product is maybe 10% penetrated, and its CDB product less than that.
The reason being is that Veeva’s EDC/CDB contracts ramp over ~3-5 years, with minimal contributions in years ~1-2. So revenue from these customers has simply yet to show up in a material way in Veeva’s financials.
From a Mar-24 Morgan Stanley Conference:
“So couldn't be happier in EDC. So as you mentioned, we have 8 of the top 20. So let me explain what that means. So that's 8 top 20 customers committing to starting all new clinical trials on Veeva's EDC software…Now these are structured typically by your enterprise customers as predefined multiyear ramping ELAs. So they'll start out small in year 1, and then they'll ramp year 2, 3, 4, it's usually 4 to 5 years before you get to terminal value. So those wins, those 5 over the last 12 months have not meaningfully contributed to our financials at this point but they will as you look out and they ramp in the future.”
And from Q1’FY25 earnings:
“On the EDC side, these are typically longer in terms of duration of the original arrangement. And the first couple of years of those arrangements are relatively small. So while there will be some contribution, I don't expect it -- us to see a material contribution there, and that's included in the guidance that we've given today.”
Therefore, Veeva might conservatively have 33% of this category’s revenue already locked up, without having to win a single new customer. And with the momentum Veeva has shown, it seems likely that 50% market share is a conservative estimate looking out to 2030 (which is the percentage I’m using in my forecast — more on that below).
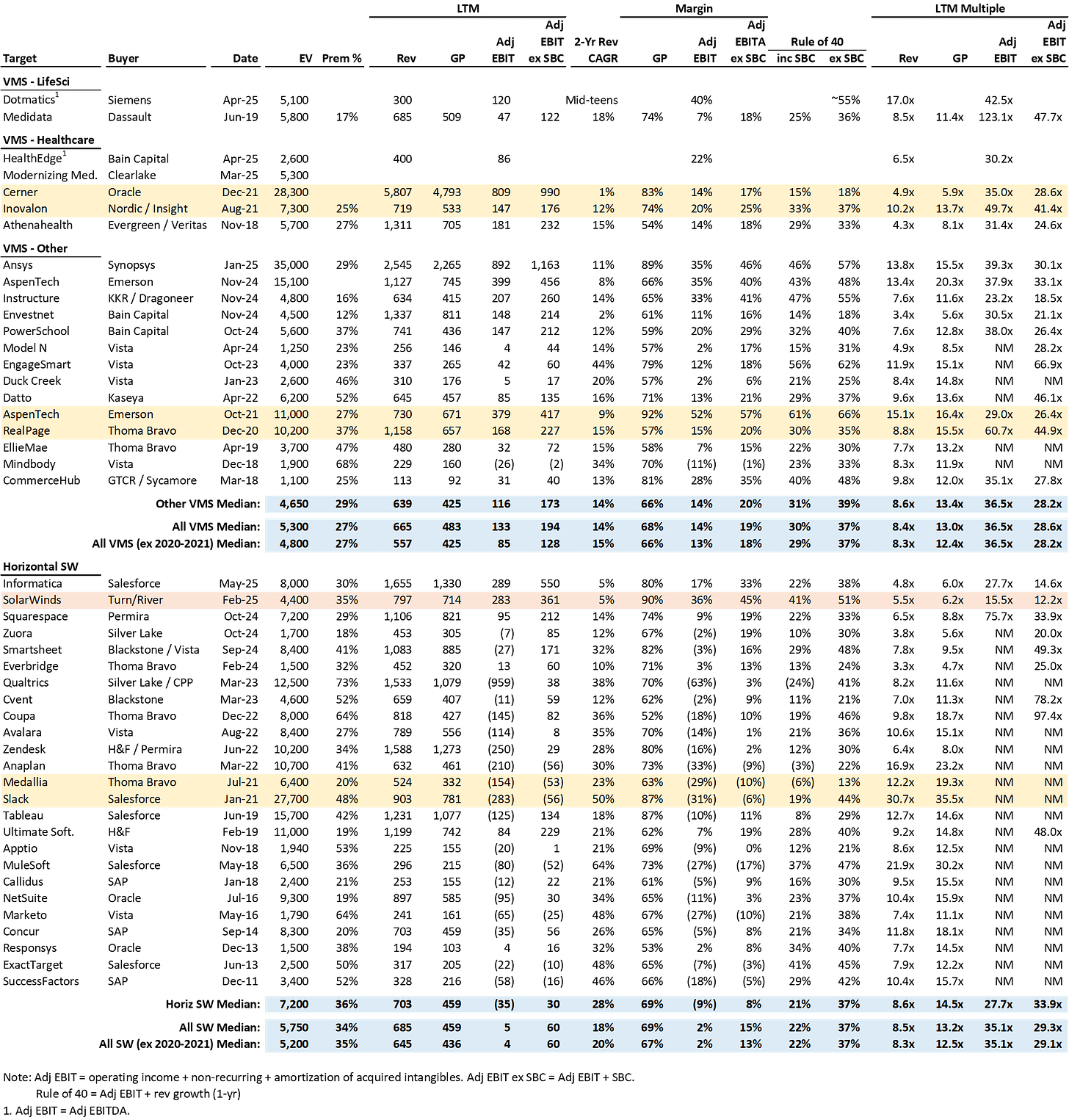
Interestingly, this is an area where, despite Veeva’s strong momentum, it is dealing with one of it’s toughest competitors in Medidata.
Medidata was founded in 1999 and its flagship Rave EDC product became widely adopted by the life sciences industry, which helped replace paper-based clinical trial processes. Medidata went public with around $100m of revenue in 2009 and grew to ~$700m in revenue before being acquired by Dassault in 2019 (10-yr revenue CAGR of ~20%) for $5.8bn (~8.5x LTM revenue).
Veeva believes it is winning vs. Medidata and others due to having (1) a better EDC product (it’s a true cloud product and includes certain important functionality that Medidata and others do not), and (2) an integrated clinical ops and data management suite.22
Gassner sees a path to dominance in EDC. From Q1’FY26 earnings:
“You touched on the eTMF, and I'll just spend a little bit there. I'm really excited about that. The 19 top 20s, and we feel like we have a pretty good path to have 20 of the top 20 on eTMF…
And I'm confident over time, actually, EDC will get there. I think — but it will take time. Today, we have 9 out of the top 20 are using our EDC. Now some of those are very early and those are multi-year ramp deals. I think we have a path to additional top 20s in EDC. I don't see anything imminent right away, but I'm sure customer success will get there over time because we have a structural advantage. People want to integrate a clinical platform from the clinical operations to clinical data management, even reaching out to the research sites.”
More color23
I estimate EDC is a ~$1bn TAM, and together with CDB represent almost a quarter of the $7bn total Clinical TAM. Management has indicated that EDC is one of its largest single product categories on multiple occasions.
RTSM / eCOA
RTSM and eCOA are key elements of the clinical suite and represent very large product categories.
According to management, RTSM/eCOA are each roughly equivalent in size to EDC. From its Q4’FY25 earnings:
“Just to give a perspective, roughly speaking…EDC is one of our larger product line areas, larger opportunities, but RTSM is roughly equivalent to EDC. And eCOA, on its own, is roughly equivalent to EDC. So these are not small areas, they're big areas.”
In fact, RTSM may actually be larger than EDC, representing Veeva’s single largest category (ex Compass). From a Dec-24 Raymond James conference:
“On the clinical side, we talk a lot about EDC and CDMS and some of our legacy products in eTMF and CTMS, but RTSM kind of quietly is our largest single product area opportunity.”
RTSM/eCOA haven’t been the strongest areas of focus yet for Veeva. Certain other apps are more “central” within clinical ops — namely eTMF, CTMS, EDC and CDB. Winning those categories are thus more helpful towards securing the entire suite. Additionally, RTSM and eCOA are areas of very high conservatism for life science customers. From Q4’FY24 earnings:
“RTSM and ePRO first, I would say, these are 2 areas where life sciences is going to be cautious. The randomization and trial supply management, that's very, very critical. If that's done incorrectly, you could have patient safety problems. You could wipe out a significant part of the investment in your whole trial. So they're going to be cautious. Companies are going to try some trials first and see how it goes, rightfully so. And in the ePRO area, the patient reported outcomes, that's a patient-facing thing. So again, they're going to be extra sensitive on that.”
But with Veeva now progressing well in the core areas of clinical ops and data, it is increasingly turning its focus to RTSM/eCOA. And as competing solutions get longer in tooth, and as Veeva’s products continue to mature, I believe Veeva will see material gains here.
One reason is that the competitive set is not strong. Unlike with EDC, Veeva management is not shy about slighting its competitors in RTSM/eCOA. Another reason is that the “suite effect” is especially motivating for customers in RTSM/eCOA because these applications work so closely with the EDC.
From Q4’FY24 earnings:
“Right now, the industry is not well served. If you look at the sort of, I would say, the professionalism of the ePRO [aka eCOA] applications out there or the RTSM applications out there, they're not of the level of professionalism of Veeva, of what Veeva is doing. Our products are getting there. So that's one thing, which is both the products and the services.
And then I think the real topper is the integration, the process integration, for example, between our RTSM and ePRO. We had a discussion last week with some clinical leaders at a top 20 pharma. And when we were discussing the integration that we will do between our RTSM and our EDC and how that affects the prescreening — the screening process and the ability to get patients into the right trials, this can be transformational.”
More support24
Today, Veeva has essentially no market share in either RTSM or eCOA. I assume by 2030 Veeva can get to 20% share in each category.
I assume the TAM for RTSM is $1.2bn and the TAM for eCOA is $1bn.
Safety
Safety is similar to RTSM/eCOA in that (1) this is an area of very high conservatism for life sciences customers, and (2) according to management, the competitive set is not strong.
On conservatism, from Veeva’s 2022 investor day:
“I would say safety is maybe the most conservative area of life sciences because of what you're dealing with. And because if your safety system doesn't operate, your pharmaceutical company can actually be shut down. You can't operate without one. So it's super, super important…
[Let's take] our pharmaceutical CRM product. Historically, companies might start in one division or one region or country. Safety system is not like that because the interconnected nature of health authorities, it's literally one day, the whole system [is moved] over.
So that's why — if you talk about the stickiest of our systems ever, it may end up being our safety system…Customers know they're making a 20-year decision.”
On the competitive set (and more on conservatism) from Veeva’s 2021 investor day:
“[Safety] is a big, complex area, and we're now in year 2. We launched into this space because there are 2 major incumbents that the industry is not satisfied with. They're looking for better execution and more innovation.
And safety is an incredibly important area. If the safety system goes down, the product stops being sold. The fact that we're bringing safety to the cloud is also important. It means our customers won't have to upgrade every time there's a regulation change, which is what they have to do today.”
More on the competitive set, from a Dec-24 Raymond James conference:
“So safety is an area that's been dominated by 2 legacy providers, one that got acquired by a larger company, Oracle, and then one is more of an independent player, both kind of legacy on-premise type systems, trying to make the move to the cloud. We all know that, that's a very difficult transition to go from on-premise to cloud-based computing.
But it's also a — so the competitive environment, it's not as strong. It's a very old legacy model, but these are also systems that are very sticky, right? Once you get that safety system in place, you actually don't want to touch it a whole lot until the pain of maintaining and supporting and upgrading becomes so great that it's actually better to move. So there's a lot of conservatism in that space.
So as time goes on, those systems get older and our product has matured. And now our product is very mature, and we've added now a suite of products around the core safety area. So it used to be just Safety and then we added SafetyDocs. And now we have products around signal detection, being able to look at data outside of the industry and draw comparisons and try to predict the safety events and also analyzing and looking at your own internal company data.
So the suite of products, the maturity of the product offering has all come to create this kind of perfect storm that will play out over a number of years, right? I don't want to kind of over-index on how fast it's going to go, but we're executing well there. I feel good about that market. It feels like we're at a nice inflection point.”
This is an area where customers are reluctant to switch, but where Veeva will have an increasingly more compelling product vs. its competitors over time. Thus Veeva should gain share. Additionally, we seem to be on the cusp of a couple potential accelerants to Veeva’s market share gains. From Q2’FY25 earnings:
“Another accelerant, I would say, is as our EDC product gets more traction and we have customers start hooking our EDC product to our Safety product, there's significant savings there, significant savings. People can be re-purposed to do other things when that connection is done, so that would be — that would get noticed.
And there may be some type of breakthrough that could happen over time, too. Remember, this Safety [product] is on the Vault platform. So it has the complete flexibility of the Vault platform, including the Direct Data API. So what type of AI things can be done on top of that AI, that Direct Data API? What type of AI things?
That will be different than can be — what can be done on the legacy, and that might cause some type of a tipping point.
I believe our strategy for Safety is quite good because we know what we're doing, and we're moving along. There are 2 legacy providers that won't be able to move along at some point and we have a structural advantage. I just don't know when that's going to start happening.”
Given (1) the benefits of integrating Safety → EDC (and Veeva’s growing number of EDC customers), (2) the outdatedness of the prevailing competitive offerings, and (3) the increasing strength of Veeva’s product (+ soon to be released AI functionality), this feels like another market where Veeva is on the cusp of inflecting market share towards 50%+ (from ~10% today per Veeva’s chart above). Adoption has been slow due to a very conservative approach from customers in this area, but I’d expect the dominoes to start falling relatively soon. Given the 2 top-20 customer wins in Q4’FY25, it may be happening now.
I estimate the entire Safety suite (including Signal, etc.) is ~$1.2BN of TAM and that Veeva can capture ~33% of it by 2030. Given they have 20% of the top 20 pharmas today, this seems very attainable.
LIMS
LIMS is another significant opportunity.
It resides within the Quality suite, integrating with QMS and QualityDocs. Veeva’s Quality suite has been taking share, and LIMS is a next step for those customers planning to standardize on Veeva across the suite.
From Q1’FY24 earnings:
“Quality is a big area for us…We set out to build that. We have the early plans of doing that for roughly 10 years ago, and it takes a long time to execute on that.
We're very excited. What's fueling the momentum there is customer success. First of all, these systems are not things you change out easily or lightly. Long implementations, and you don't do it unless you need to do it. So each customer has their own timeframe when their existing systems are running out of gas.
I would say they're not investing much these days in their legacy systems because there's broad awareness that Veeva is probably a better alternative. I do get the feeling now for most customers, they think they probably will be going to Veeva for our core established products — training, QualityDocs, QMS. And the question is when? And then there's a lot of wait and see about our new products — Validation and LIMS. ‘Hey, is that product going to be real?’
…It's a long, long replacement cycle. When we talk about a long runway for growth ahead, those are the types of things, the seeds we've planted in, things like LIMS where we don't even have our first customer.”
LIMS currently has no top 20 pharma customers — however it is an essential application in the Quality suite. From its 2022 investor day:
“We're just looking for our first early adopters there [in LIMS], that's maybe the biggest application of them all in the quality area. That's for the workflow around testing — during and after the manufacturing process — quality controls, because when you're making a medicine that goes into the human body, you better believe that's tested rigorously, right, for I think, for very obvious reasons...”
As this product matures, I expect Veeva will begin making inroads.
I estimate LIMS is a $1bn market (it’s the largest application within Quality — a $4bn market), and that Veeva will win 15% market share by 2030.
Summary
Below I lay out my application-level TAM estimates (as well as my estimated 5-year market share gains for Veeva). This is highly unofficial and built on assumptions layered upon assumptions. But I think it’s a helpful exercise nonetheless.
My current estimated Veeva market shares generally line up with the chart Veeva provided. The current revenue of ~$3bn those market shares imply lines up with Veeva’s current run rate revenue (including ~$1.5bn from commercial and ~$1.5bn from R&D).
Based on my estimated 2030 market shares for Veeva, I get to an implied revenue of ~$6.2bn in 2030. Highlighted in yellow are the key product areas discussed above. The only areas not discussed are QMS/CTMS, where like EDC, the customers have already been won (17 of 20 in CTMS; 14 of 20 in QMS) but revenue ramps over time.28 The biggest drivers of growth are EDC/CDB, followed by Safety, QMS, CTMS, RTSM, eCOA, and LIMS.
To recap, I think the assumptions underlying this analysis are reasonably conservative:
50% in EDC/CDB seems low given Veeva already has won 45% of the top 20 pharmas as we sit here today in mid 2025. A counter is that new customers ramp over 4-5 years
20% share for RTSM/eCOA seems reasonable given (1) Veeva’s momentum in the overall clinical suite, (2) the benefits of further standardizing on Veeva RTSM/eCOA and (3) the lack of competitive products in the space. A counter, again, is that these products will likely ramp over a number of years, similar to EDC
33% in Safety (across the whole Safety suite) despite (1) clear signs that Veeva market share is starting to inflect (20% of the top 20 pharmas are on the core safety product), (2) the lack of competitive products in the space, (3) the benefits of Safety integration with EDC (where Veeva is picking up share fast) and (4) the coming benefits from AI functionality (that competitors will very likely struggle to replicate)
I assume relatively little market share expansion in Commercial which might be overly conservative over a 5-year window
I’m assuming no growth in overall TAM over 5 years. In theory, TAM likely expands to ~$25BN or so from just industry growth. Including AI, perhaps much more than that
While again, this exercise is highly imprecise/directional, I believe it provides comfort that Veeva can get to $6bn by 2030. These do not seem like heroic assumptions that get us there.
Beyond 5-Yr Opportunity
After getting to $6bn in revenue, in order to continue to support a healthy multiple (anything like where it currently trades), the market would need to believe that significant growth was still in store. That might seem daunting for a vertical specific provider with already high market share ($6bn in revenue against a $20bn TAM would represent 31% market share in 2030).
But I actually think there is reason to believe that healthy growth could be sustained, even beyond 2030. The drivers of which are discussed below. Importantly, we’d be able to monitor these drivers in advance of becoming “reliant” on them.
Continued Growth of RTSM/eCOA/LIMS/Safety/EDC/CDB
Based on my estimates outlined above, I have RTSM/eCOA getting to ~20% market share, LIMS to ~15%, Safety to ~33%, and EDC/CDB to ~50%.
Once these markets begin to flip, I believe they are “leader-take-most” type of markets.29 Why buy from a competitor to Veeva if (1) the product is worse, (2) they are less incentivized than Veeva to make integrations work, and (3) they seem likely of falling only further behind as Veeva takes share.
You can see this effect in Veeva’s most mature categories — CRM and eTMF. Veeva completely dominates these categories, with 90%+ share across the top 20 pharmas.
Thus, I’d expect there to be continued material runway across these products, even after 5 years.
Compass
Compass is, by far, Veeva’s largest single-product opportunity. It is also the area in which Veeva finds its toughest competitor, by far, in IQVIA.
This market is effectively pharmaceutical sales data, which is pieced together through a variety of data sources. The industry relies on this data for many of its commercial processes (and some R&D processes as well). The data helps companies do things like find prospective patients for a certain pharmaceutical product. It further helps identify the doctors who may be treating those patients so that a company’s commercial team can call on them. It's also used for things like incentive compensation (is a sales rep increasing share in a specific territory?) and sales team design (how large should a sales team be? how should rep territories be divvied up?).
Here’s an example use case from IQVIA’s 2024 investor day:
“A top 10 pharma client was launching its existing asset for a new rare renal condition. The condition is difficult to diagnose and patients are difficult to find. Given these challenges, our client wanted IQVIA's support to find accurate patient populations as opposed to relying on clinical literature alone. They also wanted us to help them identify the right physicians who would benefit most from being aware of this asset.
IQVIA deployed our AI-based analytics model to find undiagnosed patients, leveraging multiple connected data assets comprising nonidentified patient information from claims, EMR and lab data, to name a few.
We then applied IQVIA's superior AI algorithms on these data sets to lend characteristics of patients with this condition. Once the model validated patients, we enhanced it further by mapping to treating physicians. As a result, we were able to help our client identify 9,500 more HCPs with the potential to treat 25,000 more patients with this rare renal condition.”
IQVIA has been the dominant player in the space for decades. It is publicly traded — generating about half its revenue from technology/info solutions and the other half from its CRO (its actually the largest CRO in the world).
IQVIA’s CRO segment generated $8.5bn in revenue and $1.9bn in operating profits in 2024. The Technology & Analytics Solutions (TAS) segment generated $6.2bn of revenue and $1.5bn of operating profit in 2024. The TAS segment has been growing in the low single digits.
The TAS segment is comprised of software applications, consulting services and a huge number of different data products. From 2024 investor day:
“A top 5 global pharma company is licensing 630 unique IQVIA capabilities across 87 countries. Our information assets are used by thousands of their analysts, managers and executives worldwide and importantly, across multiple use cases, spanning prelaunch planning, market opportunity sizing, M&A strategies, go-to-market activities and investor relations, just to name a few.”
I estimate the total TAM for this market is ~1/3rd of IQVIA’s overall TAS revenue (or ~$2bn). That would represent ~10% of Veeva’s overall TAM estimate of $20bn. Today, Veeva likely generates <$25m from Compass despite having been focused on the category for ~5 years.30
Veeva launched Compass Patient in 2020. Five years later, in Jan-25, they introduced Compass Prescriber and Compass National. These latest two Compass products allow Veeva to, in theory, now offer a full replacement product for IQVIA. It’s the first time in decades there has been a competitive offering in the market.
Veeva is attempting to differentiate from IQVIA through:
Better patient data. Veeva claims to take a “modern approach” to data collection. Using best-in-class technology (they would argue) they are able to collect data and synchronize it from many overlapping data sources — more data sources than IQVIA, presumably. They claim this leads to richer, more accurate patient-level data. IQVIA, on the other hand, seems to rely moreso on exclusive data relationships with certain national retail pharmacies (e.g. CVS, Walgreens, etc.). While this has led to a monopoly in the space for decades, Veeva has seemingly found a way around this, and perhaps this new way has its advantages.3132
Faster data. Due to the way that Veeva collects data, Veeva is able to refresh its data daily (which IQVIA cannot, presumably). This seems like a not insignificant differentiator for certain types of customers. For a certain type of customer, the ability to act almost immediately as new opportunities arise (e.g. calling on a HCP the day after a certain diagnosis, etc.) should be important.
Integration with Veeva Software. This seems like it would ultimately be the biggest differentiator for Compass. Assuming Compass can arrive at some level of parity with IQVIA, then tightly integrating Compass with Veeva’s commercial software (where Veeva dominates) should be a clear differentiator.
Veeva has been able to win brands within two top 20 pharmas but remains far from a full ELA.
There is little risk that Veeva would replace IQVIA on an existing brand. Customers would be very reticent to disrupt the commercial functions of a brand given its limited patent life. However, because all brands do come off patent eventually, it creates a natural churn and opportunities for Veeva to jump in on the next one.
Despite the difficult road ahead in Compass, Gassner nonetheless outlined a optimistic case on the Q4’FY25 earnings call:
“We haven't seen really a trend to ELAs yet. We're still on the brand by brand. I think that trend to ELAs, large enterprise ELAs, we might be a year or so out for that, but the trend is kind of inescapable.”
Paul Shawah (EVP Strategy) outlined a similarly optimistic outlook at the Jun-25 Baird conference:
“In many areas, our product is already the very best in the marketplace. And I say it that way because there's some — it varies by therapeutic area. But there's an incumbent in place and there's a lot of effort to switch out the incumbent even though you have a better data product, and you can prove that you have a better data product. So we have to prove this brand by brand.
It's the buying cycle in life sciences that takes time. You buy one brand, you establish success and you move on to the next one. And that's the cycle that we're going through right now. And we've seen some enterprise customers start small with one and then expand to multiple brands and then expand to an ELA. That's the pattern we're looking for, and I think that's what will play out over the next several years.”
We will be able to monitor Compass’ progress over these next few years. An increasing number of brand wins, and eventually full ELA wins, would be great signs for Compass.
This market will be a slog33, but Veeva has had a tendency to overdeliver.
Commercial Reacceleration
The big recent development in the commercial suite, announced Dec-22, is that Veeva is moving its CRM off of the Force.com platform and onto its own internally developed one (the same platform that sits underneath the rest of their applications, called the “Vault” platform). As a result, all of Veeva’s CRM customers must migrate over from the “Veeva CRM” to what they are calling the “Vault CRM” by 2030.
This will likely cause somewhat of a pause in momentum on the commercial side of the business as customers focus on CRM migration.
The good news, however, is that by migrating off of the Salesforce platform, Veeva (1) will no longer pay Salesforce a royalty for use of the Force.com platform, (2) can now target MedTech customers on the commercial side (Veeva was prohibited under their agreement with Salesforce; MedTech is about a quarter of the life science industry3435) and (3) can now develop a marketing automation application (which will be called “Campaign Manager”) and a customer support application (which will be called “Service Center”). Again, Veeva was prohibited from doing this under their agreement with Salesforce.
Thus, we might see a reacceleration in commercial as we get closer to 2030. Campaign Manager and Service Center are substantial opportunities — I estimate ~$800m combined. And they are areas where smooth integrations with CRM, PromoMats and Crossix — areas where Veeva has huge market share — would make a great deal of sense. Further, the competing products in the space (Adobe, Salesforce) are not built specifically for life science, opening the door further for Veeva, which will tailor its solutions to the industry.3637
Horizontal App
At its most recent investor day in Nov-24, Veeva announced that it was in the very early stages of building a strategy in “horizontal enterprise applications.” This was pretty surprising, as this new strategy would have nothing to do with its life science business.
Not much was revealed about it until Q1’FY26, when Veeva announced that its first horizontal application will target the CRM area. Veeva also announced that the application will likely be ready for pilot customers by year end.
Here’s additional color they provided from Q1’FY26 prepared remarks:
“We aren’t looking to develop a solution that’s just incrementally better. We are taking the hard and more rewarding path to deliver fundamentally new innovation to the market. Our success will be determined over the long term, but I am encouraged by our early progress.”
Veeva picking CRM seems like the best possible outcome — Veeva has a ton of CRM expertise and Gassner knows the Salesforce product/platform like none other (he led the Force.com platform team in its early days). Veeva seems to see an opening, where by building from scratch on a newer technology platform, they can offer a product that is fundamentally better than legacy offerings.
Below is commentary from Q4’FY25 earnings which provides some additional color:
Gabriela Borges (Analyst)
“You made an earlier comment on your thinking around horizontal applications longer term and version 1 versus version 2. I'd love to hear your thoughts if you're willing to share them, what do you think the limitations are with the version 1, the cloud SaaS applications today? And where do you think version 2, from an innovation standpoint, could really shine?”
Peter Gassner (CEO)
“I don't think there's any one particular area. I think there's a core set of things [that are] better, stronger, faster, right? Accumulation of 5, 6, 7, 8 things that have been core learnings and then putting that together. And so when you put those together, it has a compounding effect, I would say that. Also, AI will change the user interfaces on the operating systems over time, not tonight, but sometime over the next 5 years, there'll be some fundamental change just in the way that the graphical user interface or the browser-based interface change things.
I think a platform that's designed with that in mind, that knows, yes, it's going to be used as a core system of record -- some of us have heard this thing from the Microsoft CEO about these system of record applications, SaaS applications are going away. I don't believe that. I don't think any of our customers are removing their SAP application anytime in the next 100 years. But there is going to be a way to use AI to dip into multiple of these applications and add value, and that's going to be a critical component. I think the newer SaaS platforms are going to be designed with that in mind because it's obvious that's coming. I think it's pretty hard to retrofit, pretty hard.”
In a cursory review of the “AI-native” CRM landscape you have solutions like Clay in particular that do seem fundamentally better than legacy options. Many of these solutions might increasingly look like humans managing AI agents (AI finds a set of prospects for a campaign and drafts introductory emails — the user then edits & sends — the AI then puts these prospects into your pipeline, tells you when to follow-up, etc.). From its commentary, Veeva seems to be sensing an opening in this type of vein.
When listening to Gassner describe the Vault platform, a lot of what they built would seem quite transferable to an AI-native platform.38 The Vault platform (1) is good at managing both data and content (documents, videos, etc.), (2) deals well with complex workflows, (3) is less relational database and more elasticsearch, (4) offers security down to the field level (which it calls “atomic security”). And more recently, Veeva has introduced the hyper-efficient Direct Data API, which will surely come with their new CRM offering.
I wouldn’t expect anything too material to come of this over the next five years, but it’s an important initiative whose trajectory should be monitored.
AI
Last but not least — AI.
As discussed below, the AI opportunity is a very interesting one for Veeva. I have AI in the beyond 5-year bucket, but I’d be surprised if we didn’t see the impact much sooner.
AI Opportunity
Veeva’s advantages re AI:
Customers are stuck on Veeva. Super sticky, vertical-specific, system of record software
Lot of rope to innovate. Significant customer goodwill + reputation for product excellence + high levels of regulation + decimated competitive landscape = Veeva will be given extensive time/opportunity to deliver on promise of AI
Extensive application suites
For agents that need to dip into multiple different applications to achieve an outcome, that should be straightforward for Veeva
Veeva will have many AI opportunities across their set of 50+ applications
Significant “proprietary” customer data assets. Trial data; all documents from trials, safety, quality, regulatory; correspondence with regulators; sales correspondence; etc., etc. All well-organized on the Vault platform in the cloud. Competitors can’t access this data and are increasingly locked out as Veeva’s market share grows.
Significant “proprietary” external data assets. All Link + Compass assets. This data is not at all easy to assemble — Veeva’s Compass offerings in particular took 5+ years to develop into just an initial version of a product (the only other group that has this data is IQVIA). Thus, like its internal data assets, these external data assets are highly proprietary in nature
Collection of great product people who are steeped in life sciences
This is a very nice set of building blocks.
Veeva’s AI journey thus far:
2015. Way back in 2015, Veeva introduced a product called CRM Suggestions. It was an embryonic form of AI, offering suggested actions to sales reps based on certain real-world patient activity.39
2019. At its 2019 investor day, Veeva discussed AI functionality it had already layered into its Safety product.40
Oct-21. Veeva released its “TMF Bot,” which used a customer’s existing data to classify documents uploaded to its eTMF application “automatically, quickly and accurately”
Nov-22. When ChatGPT was released, Veeva seemed to recognize the importance/breakthrough nature of it, but I’d say expressed a more skeptical initial reaction.41
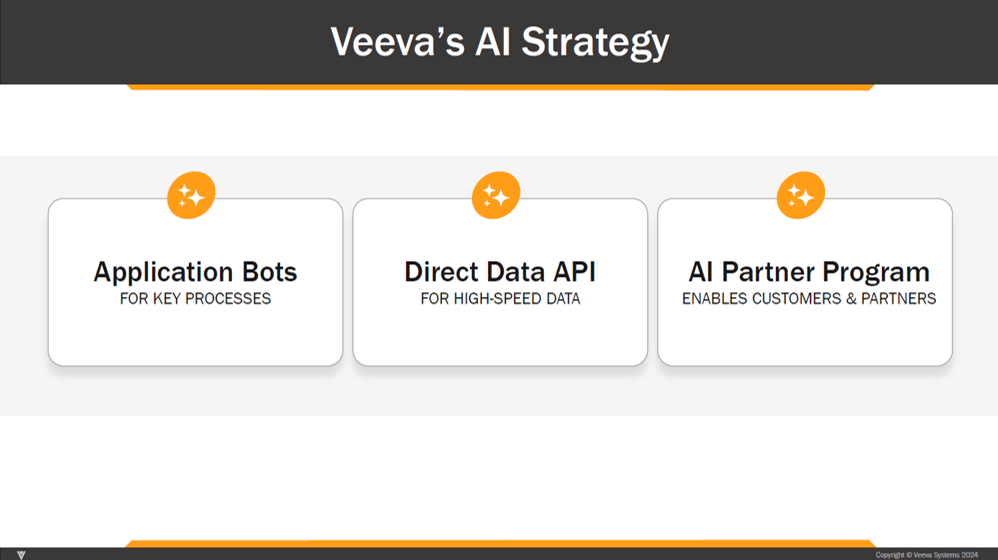
2023-2024. Over the next couple years, as the importance of AI became more apparent, Veeva’s AI strategy coalesced around a three-pronged plan: Veeva application “bots”, its Direct Data API, and an AI partner program. See below from its 2024 investor day:
Veeva application “bots.” In addition to “TMF Bot,” in Nov-24 Veeva announced “MLR Bot,” “CRM Bot” and “CRM Voice Control”. MLR Bot, which will sit within PromoMats, will perform quality checks before Medical, Legal, Regulatory (MLR) review and approval. MLR Bot will use a Veeva-hosted LLM, and accelerate MLR review by performing brand guidelines, market, channel, and editorial checks. CRM Bot embeds the LLM of a customer’s choice into Vault CRM to enable a range of tasks including pre-call planning, suggested actions, and recommended content. These “bots” will be available starting in late 2025.
Direct Data API. Veeva introduced its Direct Data API in Feb-25. The company characterizes it as a “new class” of API for easy, high speed access to Vault data without impacting application performance. Veeva claims it’s “up to 100 times faster than traditional APIs” and transactionally sound across large datasets. Through the API, a customer may “reliably extract full or incremental data to power AI applications, analytics, and system-to-system integrations.” According to management, the API took ~2.5 years to develop.42
AI partner program. Veeva launched its AI partner program in Apr-24, allowing partners to develop GenAI solutions that integrate seamlessly with Veeva applications.
Apr-25. Veeva announced it’s most aggressive stance on AI to date. Starting in late 2025, Veeva announced that all of its applications will feature “AI Agents” and “AI Shortcuts.” My limited understanding is that the “AI Agents” will be like giving an LLM access to everything in your Veeva app. However, some of the Agents may have more advanced functionality. “AI Shortcuts” seem to allow users to customize the automation of certain repetitive processes/tasks.
Illustrative AI Opportunities
Below are a few examples of how Veeva could potentially employ AI across its suite of solutions.
Example #1: Safety Signal
Veeva’s Safety suite offers “end-to-end” adverse event management. It handles AE intake, medical review, case processing, data analysis/signal detection and regulatory submissions within an unified system. Within its existing suite, Veeva has an application called Signal which helps mine the adverse event data a customer collects for “signals” that might indicate issues requiring further attention.
Applying modern AI capabilities within the Signal application would seem to present a clear opportunity for Veeva. Signal detection is the process of identifying potential safety concerns from vast, unstructured data sources such as clinical trials, EHRs, and spontaneous reports. Integrating ML tools into signal detection will improve the speed and accuracy of identifying potential safety signals.
Example #2: Regulatory Content Writer
In a clinical trial, as the final step before regulatory review, a sponsor must submit a clinical-study report. According to this McKinsey report, drafting the clinical-study report typically requires eight or more weeks to complete. McKinsey believes that GenAI-based tools can cut this time almost in half by generating an “80 percent right” first draft “from the underlying protocol, statistical-analysis plan, and tables, listings, and figures — within minutes.” Medical writers would thus be freed up to focus on sections of the report that require “a more complex clinical interpretation.”
Veeva is exceptionally well positioned to offer a GenAI-powered regulatory content writer. Veeva not only holds a huge repository of these reports in its Submissions Archive that would help train such a writer. But it also manages all trial information/data across eTMF/CTMS/CDB/etc. that would need to be accessed to draft these documents.
At its May-25 commercial summit, in his keynote, Gassner actually mentioned that Veeva was indeed working on “an authoring bot in regulatory.” I believe an AI-powered writer could be used not only in the drafting of initial reports, but also in the back-and-forth correspondence that follows between a sponsor and its regulatory agencies.
Example #3: Clinical Trial “Co-Pilot”
The McKinsey study summarizes this opportunity well:
“Gen AI can rapidly analyze vast quantities of structured and unstructured data. It is therefore a powerful study-team companion, which can share insights and suggest effective interventions to improve the outcomes of clinical trials. Several best-in-class pharmacos have already created “clinical control towers”—advanced analytics platforms that support operational decision making during clinical development by providing a single source of insights to speed up clinical trials. These AI co-pilots accelerate the trials by empowering study teams in at least three important ways: conversational AI capabilities provide tailored, actionable insights in an engaging format; smart alerts promote proactive, early interventions; and the automatic drafting of communications makes coordination with cross-functional team members more effective. These tools also promise to speed enrollment by automating analyses, proactively addressing enrollment challenges, and boosting collaboration.”
Given Veeva provides the “control tower” application in CTMS, controls the trial data in its CDB, can control the collection of the data through EDC/eCOA, etc., etc., the company is in an ideal position to provide “co-pilot” functionality. A co-pilot would need to seamlessly dip into all these applications to maximize its usefulness.
Veeva is already in the process of releasing a Pulse product for clinical trials, which would be another key building block for a co-pilot product.43
Example #4: CRM “Co-Pilot”
Veeva has already announced a CRM Bot, available in late 2025. This will feature “proactive recommendations and insights, including suggested actions with one-click execution, and content to streamline tasks and enhance productivity.”
What’s interesting here is Veeva already has its CRM Pulse product, which is an analytics engine powered by the activity of its CRM customers. Amongst other things, the pulse product can:
See errors and gaps in targeting and engagement processes by comparing to industry averages
Know which HCPs reps should see, but aren’t
Know which HCPs reps shouldn’t see
Identify the best channels and frequency for engagement
Understand field team productivity vs. the industry
These types of insights would be highly useful if made available in a co-pilot/bot product.
But another very interesting opportunity would be to tie/attribute salesforce activity to actual real-world outcomes using Veeva’s Compass products.
The Compass products provide visibility into patient and HCP activity within the U.S. healthcare system. By “closing the loop” from salesforce activity to real outcomes, Veeva could build an AI engine that would be extremely effective as well as extremely difficult to replicate by a competitor.
Path Ahead
Adding these types of capabilities to its products should only increase Veeva’s differentiation. They would make Veeva’s products better, and I don’t think they’d be easily replicated by competitors.
In fact, you could envision scenarios where these models create self-reinforcing flywheels — where greater use of say a Regulatory Content Writer makes it better over time, which then attracts even greater usage.
Materially better products — if nothing else — would in theory lead to faster market share gains.44 If you are a large pharma company that cannot use Veeva’s AI-powered authoring bot, spending weeks instead of minutes drafting clinical reports, you’ll be at a real disadvantage. It would create a strong impetus to adopt not only Veeva’s RIM suite but perhaps also its clinical ops and clinical data suites, which would need to be tapped into to draft these documents.
AI TAM
According to the McKinsey report referenced above, it expects GenAI to produce between $60-100bn in annual value for the industry. Most of this value falls within areas where Veeva is the key technology provider (all but “research and early discovery” below).
If we take the low-end estimate of $45bn ($60bn ex “research and early discovery”), and cut it by two-thirds, that would equate to a TAM expansion of $15bn. This would almost double Veeva’s current TAM of ~$20bn.
Here is a more detailed summary of potential value creation opportunities within just the clinical development function, from another McKinsey report:
Perhaps the most bullish data point for AI came on Veeva’s latest Q1’FY26 earnings call, where Gassner made the following statement:
“I'm very bullish about what Veeva can bring with AI for the life sciences industry. I think if you look over the next 3, 4, 5 years out to 2030, I think Veeva can help increase life sciences efficiency by 15% or so with Veeva AI. That's a huge number when you look at that. So I really think it will be a step change.
Why am I so bullish on it? Because Veeva has the core applications, and we're building the AI very deeply embedded in the core applications. So when we build AI, we're not building a generic AI. We're building a medical legal regulatory approval agent, a CRM agent that does pre-call planning, a safety AI agent that can transcribe pretext into a safety case, so deep AI applications. And you need the deep core applications and the AI working together.
And that's where the magic will happen. It's just very, very, very clear to me. That's what I'm excited about.”
A 15% increase in efficiency applied to a $2 trillion industry is a whole lot of efficiencies — around $300bn in theory. If we say Veeva captures just 10% of that, that would represent something like a $30bn expansion to its TAM.
While this sounds hyperbolic (and probably is?), there might be no one on earth better qualified than Gassner to understand this, and Gassner generally isn’t someone who’s prone to hyperbole.
How High Will Margins Go?
Veeva’s GAAP operating margins have improved by ~500 bps over the last ~10 years, going from around ~19% (FY10-FY15 average) to around ~24% (FY21-FY25 average). Veeva’s non-GAAP operating margins have improved by ~1,800 bps (21% → 39%), with the 1,300 delta between GAAP and non-GAAP being SBC.
I think Veeva can generate an additional ~800 bps of non-GAAP (~1,000 bps of GAAP) margin improvement over the next 5 years.
Gross Margins
Gross margins have increased from ~58% (FY11-FY15 average) to ~72% (FY21-FY25 average). That’s ~1,400 bps total over ~10 years, or ~140 bps per year.
This was a result of (1) increased subscription gross margins, driven by a shift away from the Force.com platform (or from commercial revenue to R&D revenue):
And (2) increasing subscription revenue (which carries a higher GM; see above) as a % of overall revenue:
GMs should continue to increase. Subscription revenue as a % of overall is expected to continue to increase, which will be one factor. Another will be that on the subscription side, Veeva will benefit materially from the discontinuation of the royalty it pays to Salesforce for use of the Force.com platform. As discussed, Veeva is moving its customers from Veeva CRM (built on the Force.com platform) to Vault CRM (built on Veeva’s Vault platform) over the next five years (with a hard cut-off in 2030).
Veeva still generates some ~30% of its subscription revenue on the Force.com platform (see below, from its Nov-22 investor day).
GMs are materially lower on this piece of its business vs. its in-house Vault platform. From a 2018 Deutsche Bank conference:
“The one thing that I would say as you sort of walk up the P&L from operating margin to gross margin, that you know, Karl, but it's important to make sure people understand as well, is there is a material difference in subscription gross margin between Vault and CRM, and Vault is better than CRM because of the royalty that we pay Salesforce because we built CRM on the Force.com. So the way that I think about it is we sort of have R&D dollars that are up in cost of goods sold in the form of a royalty to CRM. So CRM gross margin subs is probably in the 70, 72, 73 range where Vault gross margin is much closer to what you would consider to be pure SaaS gross margins up in the sort of higher 80s and maybe even dipping into the 90s.”
Additional support.45
If we assume 25% of current revenue is generated from the Force.com platform, and assume a 75% GM on this conservatively, then in order to back into Veeva’s overall FY25 86% subscription GM, this would imply the non-Force.com subscription revenue is generating a 90% GM. This would be in line with best-in-class subscription software GMs — e.g. Autodesk at ~91%, Adobe at ~91%.
Thus I expect subscription GMs to expand steadily from 2025 to 2030 as the Force.com royalty tapers off, and to ultimately rise to 89% by FY30 (i.e. the overall subscription GM will converge to Vault’s subscription GM).
Another factor that should benefit GMs is a return of services GMs from, I believe, trough-ish levels of late to levels more consistent with historical averages. Per the chart above, services margins were 18% in FY25 vs. a FY14-FY21 average of 23%.
Services revenue has slowed as a result of industry-wide headwinds in life sciences. Compounding this issue, the company generally hasn’t done layoffs to match supply with demand. In fact, Veeva generally does the opposite of this type of thing — seeking rather to bring in talent that’s shaken loose from competitors during such periods. Further, given the large number of Veeva-CRM-to-Vault-CRM migration projects that will be required over the next 5 years, the company might be building up its services ranks ahead of this.
I assume services margins recover to 21% by FY30 (still below long-term average).
The management team has always prided itself on generating a healthy services margin. This quote from its 2022 investor day sums it up:
“I'll also call out our services margins. Services and business consulting are about 20% of our revenue and run consistently at margins above 25%. It is common for software companies to have unprofitable services units. Veeva doesn't do that. We have discipline in our services and business consulting practices and run them at healthy margins.
Business consulting margins are expected to be somewhat higher than services margins over time due to the strategic and data-driven nature of the work.”
Another quote.46
I don’t believe they’ve changed their approach.
In all, I project services GMs to recover to 21%, subscription GMs to climb to 89%, and subscription revenue as a % of overall to increase to 87%. This results in an overall GM of ~81% by FY29 vs. ~76% today, or a ~450 bps improvement.
Operating Expenses
GAAP operating expenses as a % of revenue have gone in the opposite direction of GMs. They have increased over time, from ~40% (2010-2014 average) to ~49% (2020-2024 average).
Responsible for all of increase (and then some) has been R&D, which has increased from ~13% to ~25%, or ~1,275 bps. Meanwhile, S&M has fallen from ~20% to ~14%, or -525 bps. G&A has been flat at ~10% of revenue.
S&M
I expect S&M to continue to go lower. Unlike 10 years ago, Veeva is now the industry standard (in both commercial and R&D). Everyone knows Veeva, and they can leverage this reputation across a huge set of offerings. Here’s Gassner from Q4’FY25:
“As we get more products, as you know, the field tends to get a little bit more efficient, right? There's relationships you can leverage. Sometimes there's a broader decision made rather than fixed [smaller] decisions. So that's where efficiencies in the field come. Also, every year, and we have to keep it up, but every year, if we execute well, we develop more trust. And when you develop more trust, that translates into more efficient sales because communication is higher fidelity.”
In fact, two large pharmas (Merck and BI) recently formed strategic partnerships with Veeva, essentially agreeing to standardize on the Veeva suite wherever Veeva has products. These customers have gone “all-in” on Veeva products without virtually any need for continued S&M, in theory.
Going forward I project ~30 bps of annual leverage to S&M, which is a little lower than the historical trend.
R&D
While R&D has increased dramatically as a % of revenue over time, I think it will taper down going forward.
The company has spent the last 10 years dramatically building out its suite of applications (see unofficial chart below). Many of these applications are very complex, and have yet to generate any meaningful revenue (e.g. Compass, eCOA, RTSM, LIMS, EDC/CDB, Safety).
In other words, the company has been soldiering through an extended period of heavy R&D investment. From an Aug-17 SaaStr conference:
Interviewer
“There's sort of two models that people come to in SaaS. Does revenue drive headcount or does headcount drive revenue? And it sounds like you're squarely in the former camp that you derive headcount from revenue.”
Peter Gassner
“No, no, I actually drive revenue from headcount…Any revenue that's generated in Veeva is because of people. If you want revenue four years from now, you’ve got to hire those people now. We're starting up a brand new, probably our biggest product area to date, and this year we hired 30 people [to build it]. Those people [will] produce real good revenue for Veeva four or five years down the road — that's when that [product] will be a hundred plus million dollar business, but you’ve got to hire them now…
Product has a four-year cooking cycle, right? It's like a soup - they're like oh my God I want to eat it. [I can’t until] four years from now? I’ve got to start cooking it now? That's not good! But yeah, product takes a long time.”
I don’t think this level of investment can continue simply because Veeva’s running out of major life science apps they can build. Thus, I expect Veeva to transition from a heavy “investment mode” to more of a “harvest mode” over the coming years.
You can see the signs of this transition in the company’s employee count, which has slowed dramatically over the last two years:
Another reason we should see leverage in R&D is because Veeva has developed all these products on top of the same Vault platform which creates economies of scale. Here’s Gassner from Q4’25:
“On the product side, there's more of a mathematical reason. The more products we develop on Veeva Vault, the more efficient we get. The more economies of scale we drive out of the platform, the better the platform gets and the more economies of scale. So we have a platform. If we have 20 applications on that platform, it's an efficiency of one thing. If we have 40, it's an efficiency of another. And if we have 80, it's a whole efficiency of another thing. So that one is just more mechanical on that side.”
Not so long ago, the company bragged about how the Vault platform allowed for a mid-teens R&D (vs. 25% today) despite material ongoing innovation.47
Lastly, while I’d expect Veeva to be more of a laggard regarding CodeGen app adoption, I imagine there will certainly still be some efficiencies to be gained from these types of tools over the next 5 years. MSFT reportedly is already generating ~30% of its code from such tools. In a more competitive space, such gains might be competed away — but I think Veeva should capture a healthy portion of any AI driven efficiencies on account of its huge competitive moat.
Going forward I project ~20 bps of annual leverage to R&D.
Overall
Overall I’m forecasting ~600 bps of improvement in gross margins and ~400 bps of improvement on opex, or ~1,000 total (on a GAAP basis; ~800 on a non-GAAP basis). This results in GAAP operating margins of ~35% by FY31 vs. ~25% today.
This would also take non-GAAP operating margins up to 50% by FY31, a level where basically no other comparable companies operate. Adobe is currently at 47%, Intuit is at 39% and Autodesk is at 35%. Looking at the list of acquired SaaS co’s, Instructure had a 41% margin, SolarWinds a 45% margin, Ansys a 46% margin and AspenTech a whopping 56% margin. Virtually everyone else is well below those levels.
So I don’t feel great about projecting a 50% margin. But I also don’t think its overly optimistic. The rationale behind the 600 bps increase in gross margins is straightforward. I would be very surprised if there’s not continued operating leverage in S&M. The only lever really to quibble with is the R&D line. But there again I think I may be overly conservative — the company has ramped spend dramatically to build out its suite, and now that suite is largely complete (and the suite is starting to generate real revenue). It could be possible that AI development efforts delay the onset of this, but I think it’s coming.
The company has always operated differently. Gassner reminds me of this famous Buffett quote:
“Our experience has been that the manager of an already high-cost operation frequently is uncommonly resourceful in finding new ways to add to overhead, while the manager of a tightly-run operation usually continues to find additional methods to curtail costs, even when his costs are already well below those of his competitors.”
Buffett wrote that about Gene Abegg of Illinois National Bank way back in 1978, but I think it applies to Gassner today.
He believes in frugality and he believes in lean teams (that they actually go faster and ultimately produce better work than larger ones). He simply does not spend money unless he sees a commensurate return.484950
Lastly, it should be noted that for Q1’FY26 earnings, Veeva posted its highest ever quarterly non-GAAP operating margin of 46% (a jump of a full 3 percentage points from Q4’FY25). While management expects margins to come in some over the course of FY26, it’s certainly a data point in support of Veeva’s ability to expand margins from here. Outperformance was driven by higher GMs for subscription and services, as well as operating leverage across all of R&D/S&M/G&A — all categories, in other words.
Valuation
Key to completing this story is understanding a likely multiple range for Veeva over time.
Today it trades around ~14-15x LTM revenue / ~19x LTM gross profit.
This is up materially from just a few months ago, when it traded in the ~10-11x LTM revenue range.
Since 2023, Veeva’s traded in the 10-15x range, after getting as high as ~35x during the ZIRP-era.
Trading Comps
A set of “high-quality” software comps currently trades around ~11-12x LTM revenue / ~14-15x LTM gross profit.
Looking at multiples over time across the software industry, the average multiple is actually lower today than in 2017.
However, we can see the dispersion of multiples has become much greater, with higher growth software companies (as well as the top ten multiples in the space) commanding much higher multiples today than in 2017. I’m not sure why this wasn’t the case in 2017, but clearly higher growth should warrant a premium, all else equal.
Below is an interesting chart from Goldman showing revenue multiple vs. growth, but dividing the universe into >15% FCF companies and <15% FCF companies.
We can see a huge difference in average multiples between the two cohorts. On the right side of the slide, we can see that the average multiple for the <15% FCF company has been cut in half over the last 3 years as a greater premium has been placed on profitability since the COVID period.
Very simply, the more profitable you are, and the higher the growth you have, the bigger the multiple you will receive, all else equal:
Veeva doesn’t warrant it’s current ~13-14x NTM revenue multiple based on its growth rate — far from it. Based on its growth rate alone, it might warrant a ~5-10x multiple. However, Veeva is extremely profitable (~40% FCF margin) with high predictability and defensibility. So somewhere 10x+ seems to make sense so long as it can sustain relatively healthy growth.
Here’ what our “high-quality” software comp set looks like, charting (1) revenue multiple vs. Rule of 40 and (2) revenue multiple vs. revenue growth:
These charts support the case for a 10x+ multiple given Veeva’s set of metrics.
M&A Comps
Below is a set of M&A comps for Veeva.
The median transaction for this set is 8.5x LTM revenue and 13.2x LTM gross profit.
Charting revenue multiple by Rule of 40, we see a similar correlation to our trading comps. According to this chart, Veeva’s 58% Rule of 40 would warrant a ~12-13x revenue multiple (vs. ~15x according to the trading comps).
If we chart revenue multiple by revenue growth, Veeva’s 16% growth rate would warrant a ~8x-ish revenue multiple (vs. ~12x according to the trading comps).
Where Should Veeva Trade?
Veeva is an outlier in that, should margins expand at around the rate I expect, in 5 years a 10x LTM revenue multiple would equate to a ~21x LTM non-GAAP EBIT multiple (and ~18x forward).
So Veeva, at that point, might look fairly or a little richly valued on a revenue basis (depending on how fast it’s growing), but on an EBIT basis would look quite cheap for a high-quality SaaS co. A 21x multiple would be far below (approximately half) of where the median comp trades today. The only comps that come close to trading at that level are Adobe (17x) and Salesforce (19x). Adobe is dealing with real existential issues (GenAI, Canva). Meanwhile Salesforce’s growth has slowed to the MSD range as it wrestles with an aging technology platform and encroaching competitors.
A ~21x LTM multiple would require taking the company with perhaps the best Rule of 40 in five years (likely 60%+) and assigning it a bottom decile EBIT multiple. Only the emergence of a real existential threat and/or a slowing of growth into the MSDs would, in theory, lead to something like that.
Thus I think a 10x LTM revenue multiple has a layer of conservatism to it, and I use it as my base case below.
5-year Model
Trying to put this all together, below is a simple 5 year model. It assumes:
$6.2bn of revenue by CY2030 (implies a 15% 6-year revenue CAGR)
Overall GM rises to 81% (from 75% today)
Total GAAP opex as a % of revenue drops from 49% to 45%, driven by modest operating leverage across R&D, S&M and G&A
Exit multiple of 10x LTM revenue / 21x LTM non-GAAP EBIT
At its current PPS of ~$280, this results in a ~1.4x MOIC by 2030 and an ~8% IRR. Not exactly exciting…
However, I do like Veeva a lot at lower prices. Below are sensitivities outlining returns as a function of starting PPS and exit multiple. I’ve highlighted scenarios that theoretically produce 15%+ IRRs.
Based on above, I think Veeva starts to get interesting in the $220-230 range, and really interesting below that.
Of course, if you are a strong believer in the AI opportunity (or some other part of the story, or think it will trade at a higher multiple, etc.), then perhaps you can like it north of $220-230.
Management + Ownership
Probably the best indicator of management team strength is past performance. And this is a team with great past performance.
Some of their accomplishments to date:
Grown revenue by 13x since its IPO in 2013, a CAGR of 26% over 11 years
Achieved a GAAP operating margin in the mid-20%s and a non-GAAP operating margin in the low-40%s, both best-in-class vs. software peers
Achieved this while investing heavily in a host of new products. Picked the right markets.51 Set the company up well for its next leg of growth
Executed well on M&A. Acquisitions have been strong strategic fits, moderately priced and have performed very well
Established a strong corporate culture focused on customer success and product excellence
Built a ~$50bn market cap company on just $7m of VC capital
Peter Gassner
Gassner seems to have a real nose for opportunity, even before Veeva. From Veeva’s 2014 investor day:
“I started my career as a software engineer at IBM Silicon Valley Lab and then Almaden Research Center programmer. I was developing software on the mainframe, actually.
And then the client/server market started to emerge, and I started to see this client/server market. And I went to PeopleSoft in the early days and I built up that team. I was the General Manager of the Technology Group, PeopleTools and the Chief Architect, and we've built probably the leading platform of its day. And there were so many applications built on this platform. That was the foundation for all of PeopleSoft, and that was great, great for me. And I look back on that 9 years and I think that was a good one. I have a spark in my eye, and that informed my idea of what customers -- what employee success is. That was employee success for me at that time.
But then I started to see, hey, this cloud thing was coming, and it was going to revolutionize everything and I wanted to be a part of it. So I joined Salesforce.com early when it was a small company, a private company, to build up that platform, the Salesforce.com platform, and take it to the enterprise market. And so that's what we did.
And then about 2006, I started to think about industry-specific software that, man, this cloud thing is great, but nobody is bringing cloud to these industry-specific problems. They are totally stuck in client/server, and these industry-specific problems are hugely important to customers. So I started thinking about that and realizing that would be the next wave of things, and that you could fundamentally change things in industry-specific software, do it in a different way, move them to the cloud, maybe mix some data in there. And that was what founded Veeva.”
I used to work with an investor who liked CEOs with the “money gene.” Gassner’s background reads like “money gene” personified, jumping on important trends early and joining the leading companies therein.
However, Gassner is getting up there in age at 60 years old. My sense is that he has another 5 years left in him, at least. But I don’t know that we should expect 10.
For now he seems fully committed. He’s still in the CEO role. The company is still innovating at a furious pace. He still owns ~9% of the company through 15.1m shares (he owned 15.2m at the time of the IPO).
Broader Management Team
The broader team generally has a good deal of tenure with Veeva. The group below has an average of ~10 years with Veeva.
Paul Shawah (EVP Strategy). Joined Veeva in 2011 (~14 year tenure). Former SVP of Commercial Cloud Strategy at Veeva. Previously Director for Life Sciences at SAP and, before that, Siebel
Jim Reilly (President, Development Cloud). Joined Veeva in 2015 (~10 year tenure).
Tom Schwenger (President, Chief Customer Officer). Joined Veeva in 2019 (~6 year tenure). Previously Sr MD at Accenture focused on Life Sciences for 18 years
Nitsa Zuppas (President, Chief of Staff). Joined Veeva in 2013 (~12 year tenure) and was formerly Veeva’s CMO. Former VP of Marketing at Siebel for 8 years
Avril England (General Manager, Vault). Joined Veeva in 2013 (~12 year tenure). Former Product at PeopleSoft
Brian Van Wagener (CFO). Joined as CFO in 2024. Was formerly with Veeva from 2017-2023 as Chief of Staff to CEO and VP of Global Sales Operations.
Erik Smith (SVP, Global R&D and Quality Sales). Joined Veeva in 2013 (~12 year tenure).
Laura Moran (President, Global Commercial). Joined Veeva in 2021 (~4 year tenure). Formerly a Partner at McKinsey
Notable departures:
Matt Wallach (still on BoD). Co-Founder and former President from 2007-2019. Now minority owner of the Philadelphia Union. Prior to Veeva was the GM of Pharma & Biotech at Siebel Systems
Frank Defesche. Multiple roles at Veeva from 2008-2023 including SVP of Global Customer Services and SVP of Vault CRM. Jumped ship to Salesforce in 2023 to apparently lead their life sciences CRM effort. According to ex employees, he is a strong leader, but came up on the services side. Now in charge of product at Salesforce, this is perhaps not his #1 skillset.
Timothy Cabral (Still on BoD). Former CFO from 2010-2020. Currently serves on a number of BoDs, including Veeva, ServiceTitan, Doximity
Jennifer Goldsmith. Former SVP of Veeva Vault from 2010-2019. Left to become Chief Strategy Officer of Instructure, before founding Tendo (a healthcare-focused technology company) in 2020
Henry Levy. Former Chief Strategy Officer and President of R&D and Quality from 2016-2023. Now President of Clarivate, a publicly-traded data/analytics/technology provider for academia, government, life science, healthcare and IP management.
Brent Bowman. Former CFO from 2020-2024. Now CFO of PowerSchool.
Veeva Culture
Veeva’s official core values are:
Do the right thing
Customer success
Employee success
Speed
Here is Co-Founder Matt Wallach describing how Veeva reinforces its cultural tenets (Strategy at Scale podcast, 4/8/25):
“We review before every big meeting. We review it on the company call every quarter. We review it before every Board meeting. We don’t just list them out the way I just did. We talk about them — and why they’re still important. And when you communicate clearly for many years and you ‘walk the walk,’ then people believe it. And it’s not ‘cool’ to do something that’s bad for customers at Veeva. It’s not ‘cool’ to do something that hurts another employee. It’s not ‘cool’ to drag your feet, and to wait until the last minute or to slow the team down. That’s just not how we do it.”
Here’s the full quote, which is great.52
Other aspects of the culture, from what I can glean:
Customers > employees > shareholders. This is how they prioritize decision making. I believe this is the right order!
Each product area operates relatively autonomously. This might sacrifice some margin, but it leads to better products & innovation and better employee morale.5354
“Execution matters most”. Emphasis on speed / toughness / accountability555657
Frugality. Spend it like you own it
Can’t argue much with these.
Here’s a quote from a Nov-24 Tegus call with a Former VP of CRM Strategy at Veeva:
“Veeva is a very tough and unusual culture also. Either you love it or you hate it. A lot of people come in and they'll go right out the door in three months. That happens quite a lot. If you stay, you stay forever. You have people like me, I stayed forever.”
Perhaps some things to worry about, according to a sampling of Glassdoor reviews:
Gassner seems to strike fear into his employees (a healthy amount of fear borne out of accountability is not a con at all, I would argue)
Gassner supposedly does some strange forms of micromanagement, like banning soda from team meetings
Pay scale seems on the low side. Lean teams + low pay scale = super normal profits. But can they retain their best people?
Ownership
While %s are low for insiders (other than Gassner), the corresponding dollar amounts are meaningful.
Management Compensation
All of Veeva’s named executive officers (NEOs) earn the same base salary of ~$450k. NEOs also earn annual RSU awards equal to 125-375% of base that vest quarterly over one year. They also earn stock options equal to their number of RSUs granted but multiplied by a factor of 3-4x (these vest annually over 4 years and carry a strike price equal to the PPS at the time of the grant).
Peter Gassner earns the same base of $450k. Gassner does not earn annual RSUs or options. Rather he is granted 5-year option packages. In Jun-24, he was granted a package of 2.65m options with an exercise price of $236.90 (equal to the 52-wk high at the time of the grant — which was significantly higher than the closing price at the time). The package vests annually over 5 years and also carries a mandatory holding period of an additional 2 years post vesting.
Acquisitions
Veeva has a strong track record re acquisitions. They’ve been smaller, reasonably priced and good strategic fits. And they’ve generally performed very well.
Zinc Ahead was a tough initial competitor of Veeva’s in the commercial content management space (i.e. a competitor to Veeva’s PromoMats product). The acquisition brought over 120 customers (40 net new) and established a stronger presence in Europe (Zinc was based in the UK). The acquisition accelerated the progression to one industry standard across commercial content management, helping Veeeva avoid a battle royale between two high-quality competitors. It was done at a very reasonable price of ~4x run-rate sales.
Crossix has performed very well post acquisition. In Veeva’s Q1’FY26 prepared remarks, they stated that Crossix was generating “more than a $200 million run rate today,” which is about ~3x larger than when it was acquired ~5 years earlier. Veeva also mentioned on the call that Crossix was growing north of 30%. I believe growth has actually inflected upwards of late at Crossix (based on disclosures at Veeva’s ‘22 investor day, Crossix grew at a ~20% CAGR from 2019-2022). Veeva believes Crossix “has grown to become the standard for marketing analytics in life sciences.” Veeva’s suite of Compass products have also been built on top of Crossix’ technology platform, which was the vision at the time of Crossix acquisition. In other words, Crossix has created very significant value for Veeva — it’s been a home run.
Veracity Logic provided Veeva with its initial RTSM solution. Veracity tripled its revenue less than 1 year post acquisition.58 RTSM will be a key category for Veeva over time.
From its 2023 investor day:
“Most acquisitions don't work out. That's the fact. They all work out for the bankers and the lawyers, it all works out. But mostly, it doesn't work out. Those are the facts. And if you look at it, it's — I think it's somewhat to lack of discipline. So we're very disciplined. All of our acquisitions have worked out.”
Other Interesting Nuggets from Management
On the high predictability of developing great products59
Key Investment Risks
Steep Multiple
Veeva currently trades at ~15x LTM revenue, ~20x LTM gross profit and ~55x GAAP EBIT. That’s pretty steep for a company expected to grow less than 20%, even for a business of Veeva’s quality.
Not so long ago Veeva traded in the ~10-11x LTM revenue range, which I think is a level that could be sustained longer term. At ~15x, I think you’d need to bake in a fair degree of multiple compression which will weigh on returns.
Near-Term Growth Slower than Expected
While I believe Veeva will gain significant share in R&D, it could happen more slowly than anticipated. In certain areas like Safety / Quality / Regulatory, customers may be happy to use incumbent solutions so long as they continue to work. While Veeva’s products may be objectively better, they may not offer enough cost savings, etc. to incentivize strong levels of near-term migrations — it could be a slower drip instead.
However, as mentioned earlier, AI could act as an accelerator of sorts. For example, an AI-driven regulatory writer could shave weeks off the drafting of certain regulatory reports. In the Safety suite, AI could be used to drastically reduce the manual work needed to prep adverse event cases for review. Such functionality, if it does come to fruition, would make it tougher for customers to postpone a move.
Longer-Term Growth Drivers Break Down
While I believe growth in Veeva’s R&D suite will very likely sustain attractive levels of overall near-term growth, some of Veeva’s longer-term growth drivers are still very much “question marks” at this point.
AI. AI might not materially add to TAM. We don’t know yet how much Veeva can charge for its AI functionality. Just enough to make up for lost seats? Way more than that?
Compass. Veeva could fail to take meaningful share from IQVIA in its Compass business. IQVIA has been building its offerings for over 3 decades and enjoys a monopoly-esque position. Its products benefit from huge switching costs.
Horizontal CRM. This is a start-up targeting an entirely new set of customers. While Veeva has tremendous expertise in platform building / CRM / etc., this is a highly competitive space and a departure from Veeva’s tried and true model of selling into life sciences.
These initiatives should be monitored closely. To sustain teens levels of growth beyond the current wave of R&D-led growth, at least one of AI or horizontal CRM would probably need to really hit.
Is Veeva’s TAM Really $20BN?
Here is Veeva’s TAM slide from its latest 2024 investor day:
As you can see, they are simply assuming that Veeva’s TAM is 1% of the overall market for biopharma + medtech. Not exactly the most rigorous analysis you will see. So can we trust this TAM figure?
If we go back to 2022 (and earlier), Veeva actually did a bottoms-up TAM analysis based on its then current line-up of products. As we can see below, in 2022 it calculated its TAM as $13BN using this methodology.
In 2023, Veeva switched to a top-down calculation, and included TAM that wasn’t serviceable by its then-current products, but could conceivably become serviceable over time.
We can see above that in 2022, the Commercial TAM (of $6bn) is approximately equal to the $7bn that is assumed for Veeva’s 2024 Commercial TAM. Further, the delta could easily be explained by the additions of Campaign Manager and Service Center.
The expansion to the R&D TAM from 2022 to 2024 is more of a leap, growing from $7bn to $13bn. And there were no major product releases post 2022 that could potentially explain this. Perhaps Veeva decided these R&D categories were larger than they once thought, or more likely they think there is just significant additional whitespace in these categories (or both).
Either way, the R&D TAM of $13bn feels a little squishy, based on this. That could be a source of risk, leading R&D growth to slow sooner than expected.
One helpful reference is to go back and look at Medidata. At the time Medidata was acquired in 2019, the business generated ~$700m of revenue with ~2/3rds of that coming from its EDC product.60 It also claimed that it had a market share of “probably more than half of all electronic trials globally.”61 If we assume its market share was 60%, then the entire EDC market would have been ~$750m as of 2019. If we assume a 5% CAGR, the market would be ~$1bn today, which is what I assume in my TAM analysis.
We can then back into some of the other R&D, product-level TAMs. If the EDC TAM is $1bn, that means the eCOA TAM is also ~$1bn and the RTSM TAM is maybe a little bigger than that. Given the commentary around LIMS, I’d expect it to be a similar size as well. This adds more comfort around the $13bn figure.
What would really add comfort around TAM, however, would be a clear expansion of it through AI. As discussed above, Gassner shared on Veeva’s most recent earnings call that he believes Veeva AI can make the life sciences industry 15% more efficient over just the next 5 years. If anything like that comes to fruition, and if Veeva is able to capture just a tiny bit of it, it would lead to very material TAM expansion.
Gassner is Getting Older, Wallach Already Left
At 60 years old, I would guess that Gassner probably has more than 5, but less than 10 years left in the CEO role. Co-Founder Matt Wallach already left in 2019.
My feeling is that Gassner is both very excited about the prospect of embedding AI throughout Veeva’s suite of offerings as well as taking on Salesforce with a horizontal CRM built on an entirely new platform. My understanding is that Gassner led the Force.com platform team for Salesforce as well as the PeopleTools platform at PeopleSoft. After building another platform in Vault, he now has a chance to build a fourth from scratch, leveraging everything he’s learned to date.
I expect he’ll want to see both of these initiatives through at least their initial phases over the next handful of years.
Poorly Executed M&A
Veeva has built up a war-chest of $6bn in cash and generates over $1bn more in excess cash flow annually. Veeva mentions M&A most often as a potential use for that cash. So poorly executed M&A is a risk.
However, Veeva has proven to be a very careful acquirer.
Management highlights the Crossix deal as the type of transaction they seek. In Crossix’ case, on top of it being (1) an industry-leading solution (2) at a reasonable price, (3) that was a strong strategic fit, (4) with a great team, it could also be (5) cross-sold to Veeva’s huge commercial customer base, and (6) used as the technology platform for its Compass products. In other words, it was clear that Crossix was going to add a ton of value — a “no brainer.”
With this type of discipline, if Veeva does acquire, I think it will likely by an intelligent use of capital.
Veeva “Fatigue”
It’s reasonable to think that customers might be experiencing some Veeva fatigue. How smart is it to go “all-in” with one provider across your entire commercial and R&D functions? Is it smart for the industry to consolidate on one vendor and potentially stifle innovation and/or materially reduce bargaining power?
Factors that should help Veeva overcome this: (1) they have done right by customers in the past and have gone as far as converting to a PBC to ensure that they will do right by their customers “forever,”62 (2) their products continue to be industry-leading, and (3) buying all your applications from one vendor (and having them integrate seamlessly) is actually an ideal scenario, all else equal.
In fact, there has been a string of customers going “all-in” on Veeva — essentially the opposite of Veeva fatigue.
Merck. In Nov-22 Merck announced a 10-year strategic partnership in which Merck will “take a Veeva-first approach to new industry-specific software and data, selecting Veeva products when they are fit for purpose.” In exchange, Merck will receive a “strategic pricing approach” and will have input into Veeva’s product roadmap. Essentially Veeva is now in the highly unusual position of being the decider of whether its products are ready for Merck, not the other way around
Boehringer Ingelheim. In Mar-22, BI announced the “One Medicine Platform,” an initiative that involved going all in on Veeva across clinical ops, regulatory and quality. This platform went live in Mar-25
Another top 20 pharma went “all-in” in Q4’FY25 across clinical ops and data.
Losing CRM Customers in its Vault CRM Migration
Veeva seems to have lost at least two of its top 20 CRM customers to Salesforce as part of the Vault CRM transition. The first was mentioned at a 12/11/24 Raymond James conference. Then on 5/19/25 it was announced that Takeda was leaving Veeva for Salesforce.
On the positive side, five top 20s have committed to the Vault CRM to date — GSK, Bayer, Novo Nordisk, Boehringer Ingelheim and Astellas.
My understanding is that Salesforce bought IQVIA’s life science CRM solution named OCE in Apr-24, giving Salesforce an initial offering in the space (an updated product under the Salesforce umbrella has yet to be released). It does seem possible that by using OCE as the basis for its CRM, and by allowing IQVIA’s data to flow seamlessly through the product (IQVIA prohibits this on Veeva’s CRM), Salesforce would have a viable path to compete against Veeva.
However, I don’t foresee a material exodus to Salesforce. These initial losses were likely sweetheart deals from Salesforce to secure pilot customers, and they can only offer so many of these. I think the mountain to climb for Salesforce is very steep given (1) the fact that Salesforce would need to develop a whole suite of life-science specific commercial applications on top of just a pure CRM to really compete toe-to-toe6364, and (2) Salesforce has never been great a delivering vertical software and likely lacks the operational capabilities to pull something like this off.
AI-Native Competition
It feels like the consensus is shifting on incumbent SaaS vs. AI-natives. I think many/most believed initially that many incumbent SaaS providers would do just fine through the AI transition (data moats, switching costs). Now it seems those on the leading edge are warming up to the idea of AI-natives taking on SaaS incumbents across all categories.
Pat Grady of Sequoia said recently that after some initial skepticism, the firm has gotten “religion” around the idea of AI-natives being able to compete effectively in the traditional SaaS categories of CRM, HRM, ITSM, etc.
While something to monitor, I still think Veeva’s moats are too formidable for an AI-native to go toe-to-toe with them anytime soon in life sciences.
Future AI-Driven Tectonic Shifts
While not something I’m worried about near-term, over a long enough timeframe, could AI disrupt the very processes on which Veeva was built? For example, what happens if all doctors are replaced by AI — how do you sell drugs to an AI bot? What would happen to Veeva’s commercial suite? What if medicines become so personalized or our understanding of the human body (and the effects drugs have on it) becomes so advanced, that clinical trials are de-emphasized in some material way?
Market Overview
Pharma Industry Growth
The pharma industry is projected to grow in the 6-7% range over the next decade:
Fortune Business Insights: $1.2tn in 2024 → $2.2tn by 2032, a CAGR of 7.1%
Cervicorn Consulting: $1.8tn in 2025 → $2.8tn in 2033, a CAGR of 5.8%
Towards Healthcare: $1.6tn in 2023 → $3.0tn by 2034, a CAGR of 6.2%
Nova One Advisor: $1.6tn in 2024 → $2.7tn by 2033, a CAGR of 6.1%
Key drivers include:
Aging populations and chronic diseases — more patients with diabetes, cancer, heart disease, etc. Older folks (65+) require orders of magnitude more spend on healthcare than young people, and the 65+ population globally is expected to more than double by 2050 (from 700m today to 1.5b in 2050)
“Pharmerging markets” — middle-income regions (China, India, Brazil, MIST countries) are expanding healthcare coverage and drug access
Growing pharmaceutical pipeline — in 2013, there were approximately 6,000 drug candidates in active clinical development worldwide. By 2023, this number had increased to over 21,000, a 250% increase. This expansion reflects intensified research efforts, particularly in oncology, rare diseases, and novel therapeutic modalities like gene and cell therapies
GLP-1s — injectable or oral medications that mimic a hormone (GLP-1) released by the gut after eating. These drugs produce better blood sugar control and significant, sustained weight loss. Analysts project that the global GLP-1 market could reach ~$100 billion annually by 2030, making it one of the largest therapeutic categories ever
A growing pharma market will help Veeva, all else equal — more drugs to be trialed, manufactured, sold and monitored
Are We Entering a Golden Age of Drug Discovery?
It doesn’t seem unreasonable to think that the industry could be at an inflection point regarding drug discovery, driven by new, colliding technologies:
Gene sequencing — the process of determining the exact order of DNA building blocks in an organism. The cost of sequencing has dropped from ~$100 million in 2003 (when the Human Genome Project was completed) to under $200 today, and it has become the foundation of modern drug discovery. It enables a shift from broad, trial-and-error medicine to precise, personalized, and genetically informed therapies. CRISPR and mRNA weren’t possible before it, and it fuels AI tools that mine genetic data to prioritize drug targets and design molecules.
AI / ML — From the Economist: “In 2020, AlphaFold 2, a model made by DeepMind, an AI laboratory owned by Google, stunned the scientific world by accurately predicting the structure of nearly every protein in the human body. In May 2024 its successor, AlphaFold 3, expanded its capabilities to other molecules that make up living things: proteins, DNA, RNA and small molecules called ligands. Such models are changing drug development by reducing months of trial-and-error experiments to just hours of computation. Insilico, a startup that uses AI to develop drugs, says its software identified a new drug target and designed a molecule suitable for human trials in only 18 months and at a cost of $2.7m — a fraction of the usual time and expense required.” BCG estimated in 2024 that about 65 “AI-inspired” molecules were already in human trials as of 2023:
One would expect this number to continue to grow exponentially. A host of buzzy new start-ups (like Isomorphic Labs and Xaira Therapeutics) have raised large sums to pursue AI-powered drug discovery. Many of these AI specialists have formed partnerships with the big pharma.
CRISPR — uses molecular “scissors” to cut DNA at specific sites and edit genes permanently, correcting genetic mutations and modifying cells (e.g. for cancer therapy). In late 2023 the FDA approved Casgevy, the first FDA-approved CRISPR/Cas9 treatment (for sickle cell disease), signaling that precise gene correction was officially clinically viable. Much more CRISPR (and base-editing) testing are underway for blood disorders, eye diseases, heart diseases, diabetes, cancer, congenital blindness, H.I.V., etc. According to the NYT, “all told, some 400 million people worldwide are afflicted by one or more diseases arising from single-gene mutations that would be theoretically simple for Crispr to fix.” Just last month, the world’s first patient was treated with personalized CRISPR gene editing therapy at the Children’s Hospital of Philadelphia.
mRNA — delivers temporary instructions to cells to make proteins, helping treat diseases. From the NYT: “Drug-development timelines in previous history had swallowed whole decades; experts warned not to expect a resolution [a COVID vaccine] for years. But the mRNA sequence of the first shot was designed in a weekend, and the finished vaccines arrived within months, an accelerated timeline that saved perhaps several million American lives and tens of millions worldwide — numbers that are probably larger than the cumulative global death toll of the disease. The miracle of the vaccines wasn’t just about lives saved from Covid. As the first of their kind to be approved by the Food and Drug Administration, they brought with them a very long list of potential future mRNA applications: H.I.V., tuberculosis, Zika, respiratory syncytial virus (R.S.V.), cancers of various and brutal kinds. And the vaccine innovations stretch beyond mRNA: A “world-changing” vaccine for malaria, which kills 600,000 globally each year, is being rolled out in Ghana and Nigeria, and early trials for next-generation dengue vaccines suggest they may reduce symptomatic infection by 80 percent or more.”
Antibody–drug conjugates (ADCs) — are a class of targeted cancer therapies that combine the precision of antibodies with the potency of chemotherapy drugs — essentially acting as guided missiles that deliver toxic payloads directly to cancer cells. As of 2024, there are over a dozen FDA-approved ADCs and more than 100 in clinical trials. Pfizer acquired Seagen (a leader in ADCs) for ~$43 billion in 2023. Merck, Roche, and AstraZeneca are all heavily investing in ADC pipelines.
A more vibrant industry should again be beneficial to Veeva.
Furthermore, as drugs become more specialized/personalized, this could have additional benefits for Veeva — finer targeting of patients will be increasingly important; more sales expertise could be required to educate HCPs on niche drugs; clinical trials will likely become more decentralized (and thus more tech-enabled) as patient populations become smaller & harder to access; manufacturing/safety processes might become more complex.
Decentralization of Clinical Trials
From Fierce Pharma: “Over the past few years, factors like the Covid-19 pandemic have accelerated the adoption of decentralized — or Virtual — clinical trials. This approach leverages advances in digital technologies, data collection and logistics capabilities to allow patients to participate in clinical trials from their own homes, rather than travelling to an investigator’s site or the nearest hospital. While a decentralized approach may not be suitable for every clinical trial, data from Accenture shows that 24% of clinical trial studies now offer a home-based solution, with that number expected to grow. Considering the positive impact that decentralization can have on patient engagement, and improving patient outcomes, it’s no surprise this is a trend we see on the rise.”
It’s probably fair to say that an increasing number of virtual/hybrid trials should work in favor of technology providers like Veeva, increasing the importance of tools like trial monitoring and collaboration (CTMS, eTMF) and remote data capture (EDC, ePRO).
Pharma Industry Woes
As someone who basically hasn’t spent any time looking at the pharma space, in digging deeper I was expecting to find a relatively healthy industry. But that’s not exactly what I found.
Here are 3 pharma ETFs vs. the S&P over the last decade:
The industry seems to be dealing with a mix of (1) ballooning costs to develop new drugs, (2) a lack of new “blockbuster” drugs (other than the GLP-1s) and (3) a looming wall of drugs that will be coming off patent (that amount to some ~$200 billion in annual sales).
Add in (4) a post-pandemic slowdown, (5) higher interest rates (and a resulting “funding winter” for new biotechs), and (6) increased pricing pressure due to the US Inflation Reduction Act, which allows Medicare to negotiate some drug prices directly with pharma co’s — and you’ve got a recipe for tough times. To adapt, many have cut costs and streamlined R&D pipelines.
While on one hand, this makes Veeva’s performance perhaps even more impressive. On the other, these pressures seem like they’ll continue weighing on the industry — which is not great for Veeva.
The industry seems to be dealing with a degree of “broken-ness” to the business model, where revenue from new medicines are not paying for the costs to develop them. From a recent Sequoia presentation:
By their estimates, it currently costs $2-4bn and takes ~13 years to bring a new drug to market. However, this does appear to be an industry that’s very, very ripe for AI solutions to some of these problems.
Conclusion
Veeva is an example of owning a fantastic business at an “okay” price (assuming something like a $220-230 entry, currently).
It fits nicely with a strategy of avoiding losers while letting the winners “take care of themselves.” In other words, it seems unlikely that in a few years, you’d look back and be down materially on your position. While you could also not be up by much either, there are scenarios that would get you upwards of a 20% return — if R&D revenue hits quickly, if the AI opportunity really turns into something, if horizontal CRM breaks through, etc.
It is also a bit of a bet that AI value will accrue to the “app layer” (as opposed to the “model layer” or the “infra layer”). The “app layer” is where most of the gains from the cloud era accrued, and many expect the same to play out in AI.
Veeva is clearly hyper focused on “automating” as much as it can within life sciences. Gassner on a May-22 Acquired podcast (even before the “ChatGPT moment”):
Q: “What makes the future of Veeva an A+? What's the like scenario where it goes incredibly well paint that for us…”
A: “A+ is we really help automate this big industry, right? It's a two two trillion dollar industry and if we can help to automate it and be that trusted partner that that is essential to that industry and using that word very specifically — essential — and appreciated. There's not been anything like that before where you're automating a whole industry in a meaningful way, right? And essential. You're gonna be a life sciences company? You’ve gotta use Veeva and man you like that. So that would be a big success.”
Veeva seems firmly on the path to achieving this to some degree, with AI now the big driving force. A company that was actually able to achieve this should do quite well over time, one would think.
Important Disclaimer
This post is intended for informational purposes only and you, the reader, should not make any financial, investment, or trading decisions based upon the author's commentary.
Although the information set forth above has been obtained or derived from sources believed to be reliable, the author does not make any representation or warranty, express or implied, as to the information's accuracy or completeness, nor does the author recommend that the above information serve as the basis of any investment decision.
Before investing in a security, readers should carefully consider their financial positions and risk tolerances to determine if such a stock selection is appropriate. At any time, the author may trade in or out of any securities that are mentioned in the report as long or short positions in his portfolio without disclosing this information.
This report’s estimated fundamental value only represents a best efforts estimate of the potential fundamental valuation of a specific security, and is not expressed as, or implied as, assessments of the quality of a security or an actionable investment strategy for an investor.
“An so when a company would go all in with Veeva, we might replace 200 different pharma vendors…At most of the companies we went to, they spent more money integrating the different things that they bought than they did buying those things…So companies, the more they bought from Veeva, the more valuable it got.” — Matt Wallach, Strategy at Scale podcast, Apr-25
Discussing just the Regulatory suite:
“For regulatory, we have our RIM Suite or regulatory information management, and that's quite a mature product set. It's an industry-leading product for regulatory, but there's still a lot of legacy to replace because these are hard and difficult implementations, and the customer has to be ready to do it.
So for example, at a large pharma, we may replace 30 or more legacy systems, custom systems, packaged systems and all the integrations between them. So that's a lot of work. So there's a big mess to clean up in regulatory, and we're working our way through that. We have a great product and a great product team.” — 2022 Investor Day
Discussing just the Quality suite:
“And that's a strategic as you get — not to mention, most likely in a big pharma, this quality suite will replace probably at a minimum 40 separate applications and maybe up to 100 different applications.
And you think about how that can; a, that's why you know it takes a while to do. But also that's how you know there's tremendous efficiency when you replace that many applications.” — 2022 Investor Day
“It's underappreciated how hard it is to build an industry-specific CRM for life sciences. I mean if you think about it has to meet the regulations of Japan and Brazil and Germany, which are all different. It has to meet the needs of the state of Ohio, which is different than the state of New York. How you handle samples, how you share content in a highly regulated way, it's deep, it's hard.
And I think what you've seen, we had a competitor who tried to build on top of Force, and it didn't work out so well because it was hard. We lost a couple of customers and guess what? They came back to Veeva because they lost time. They lost time and efficiency in that move. So I think that's underappreciated how deep and complex the application is.” — Mar-24 Morgan Stanley conference
“Safety means for a pharmaceutical company, they will have a drug, somebody might report an issue with that drug. They got sick if they took that drug. They had an adverse reaction to it. They would report that to a pharmaceutical company.
And that safety issue could occur in any country around the world. The pharmaceutical company has a duty then to analyze that and actually to report it to the right health authority all around the world and also to collect what's called the ‘signal.’ They need to analyze all these different safety events coming in and try to see if they have a pattern. If they don't do that correctly, it's a big problem. [There] can be major fines.
It could result in real problems for patients as well. And it's very detrimental to the business. This is also sometimes called pharmacovigilance, this area. It's a really big, important area” — 2017 Investor Day
“When you have an event and you have health care providers that come to an event, the legislation like the Sunshine Act requires that you track all of the spend against the health care provider and aggregate and allocate that spend to individuals who come. So they come to an event and they have a meal, you're allocating event cost and the cost of that meal to that individual person. And you have to roll all that information up across all the events they go to and every other activity that you do against them across your company. If you have that data in multiple different systems, it's very difficult to bring that all together and do things like compliance reporting. So the #1 driver for doing Events Management in a more consolidated way than what this company had, is compliance.” — 2016 Investor Day
Peter Gassner discussing their emerging suite of R&D applications:
“Now if we can do that, this is really a franchise for Veeva for literally the next 30 years, at least. These are -- these systems are very sticky. Once you get them in, and they're working, you are not changing them. These are the heart of the drug development and safety process. So these are very significant applications.” — 2017 Investor Day
“We have a goal to be the leader and liked. We actually tell our customers about that and that holds us to a higher standard. ‘We want to be the leader and liked.’ And then they bring that up sometimes, like ‘hey, that's not leader and liked!’ ‘Oh god why did I tell you that?’ It's a way to be set yourself out there.” — May-22 Acquired podcast
“Now in our selling structure, we really take the long-term view of this. We're looking for 10-year-plus partnerships with customers. We're not looking for a transactional, hey, what's your sales quota this quarter, that type of thing. It doesn't play. It's not compatible. It's oil and water. You have to know what you're doing. Are you short-term transactional or are you long-term strategic? We're long-term strategic. We take that view in everything we do.
And we don't overcover with salespeople because that causes stress on your customers. If you have too many salespeople selling too many products that aren't ready, they're desperate. That causes frictions with the customer. That causes bad customer feeling. It's not good. So what we say is we don't overcover. Right amount of salespeople at the right time, definitely okay with sacrificing some top line revenue in the short term for long-term value. This is not new for us. This is always how we've operated.” — 2016 Investor Day
“With sales you know we have a philosophy that we say we don't overcover. So we want to leave topline revenue on the table. We don't want to get all the topline revenue, because if we if we push all the way for that, we will get some inefficiencies and that's not so good, but also we will get bad customer feeling. Because a desperate rep will do desperate things. And the rep's in front of the customer and they'll feel it. You don't want those desperate things do you? No, because for us we have many customers that are you know they're over well over $20 million a year customers for us. These are 20-year relationships, do we want somebody in there saying I got to close this thing this quarter because of my commission and I don't really care that you're paying us $20 million a year. So the way you do that, is you sacrifice topline revenue and you don't put too many reps out there. So then the rep is not desperate…” — Aug-17 SaaStr conference
Veeva achieves a leading payback number despite a very complex, field-heavy sales team. David Skok of Matrix Partners once laid out an illustrative chart on CAC vs. the complexity of a sale (see below). Field sales, he found, generally led to a many-orders-of-magnitude larger CACs vs. a freemium or no-touch model.
Despite this very significant disadvantage, Veeva essentially beats all it’s high-quality SaaS peers on CAC payback.
“The industry — it's not recession-proof, but very recession-resistant. This is not like airlines or hotels or luxury items, things like that, restaurants. People need their medicine in good times and bad. Medicine is a critical part of health care.
Without medicines, health care costs go up, et cetera. So the spending doesn't change too much. The segment where it is affected a little bit is in smaller private companies that if the funding environment goes down, they can't get as much funding. They have to be more conservative with their expansions.
And then I would say the last thing is the type of products we do. They are mission-critical systems of record. Good times or bad, you need your clinical trial management system. Just like you need your financial system and you need your ERP system. What you may not need is the speculative piece of technology or add on, on the top of that.
That's maybe what you don't need. And we focus really on system of records. So we have that going in favor for us. So I think a lot of things going in our favor for stability. We do best in times of relative stability.
When things go booming up fast, maybe that's not actually the best for Veeva. And when things downturn a bit, maybe that's not all the best for Veeva either. We operate the best when things are pretty stable.” — Q3’FY23 earnings
Note the category of “drug development systems” does not include software for research or “pre-clinical” use cases, but solely for the clinical trials-and-onward portion of drug development.
“So I'll call out Crossix…It's for health care marketing specific to the United States, so we can measure when there's a TV ad, for example, does that TV ad lead to what type of patients, seeing what type of specialists and getting on what type of therapy.
So that's very, very, very specialized. It's a way to understand if I'm spending $100 million on digital marketing, is it being effective or not, and monitoring and changing that. You can imagine how different that piece of software is from our CRM system — very, very different, but we're the leaders in both of those areas.” — Jan-24 JPM Conference
“We have OpenData, which is our reference data. So that's the core reference data, list of doctors, hospitals, how they relate to each other, what are the specialties, et cetera, in more than 100 countries across the globe.
That's used for compliance in sales and marketing. We're going to extend that to the clinical area that's to be used in operating your clinical trials, who are the clinical research sites and investigators? So this is the encyclopedia rather than each pharma company or biotech company creating their own encyclopedia. So that's reference data.” — Jan-24 JPM Conference
“Link is deep data…a large corpus of data, complicated data model curated by Veeva, a curated data model with a very specialized interface on top of it, user interface. So you could consider it sort of something like a Bloomberg Terminal. The first and most advanced Link application is called Key People. That's an application that lets our pharmaceutical companies that are our customers keep track of the 1 million or so key opinion leaders around the world and follow them and what are they doing and who are the emerging key opinion leaders and quickly understand what they're doing. So sort of like a curated LinkedIn on steroids, if you would say, that's very, very specific to life sciences.
We have a few thousand people, data stewards and machine learning algorithms, that are curating this type of data every day. They're not our employees, they are contractors, and it's a balance of humans, very specialized humans doing specific tasks and machine learning automation. We're going to extend Link to the clinical areas as well. You'll see that theme in the data we started in the commercial area. We're going to extend to clinical.” — Jan-24 JPM Conference
“This is an example of the shift that's happening in the industry from where the primary set of data used to be from the electronic data capture. Literally, the patient going into a research site the researcher measuring something about the patient and entering it into a system, and that was the vast majority of data to run a trial. That has changed, as you know, with everything you just described, that patient being more distributed, not having to go into the office, doing wearables, maybe doing self-reported data about -- that they don't even have to go into the clinical research Stage IV and doing that in a very distributed, decentralized way. But also their lab work and imaging and all of the other things that happen around that patient are critical to the clinical trial.
And Veeva is taking a different approach. Instead of just doing the EDC, which is 1 -- it's an important part of the data, but it's not everything. We are connecting, we're helping to collect data from these other sources like patient-reported outcomes like data from a wearable, for example, and bring all that data together and mash it together to make it easier to analyze because the results of the trial are based on the full view of all of the data that you need. And today, that's very, very hard to do, especially if all you do is electronic data capture. So you mentioned CDB, the clinical database, it's bring -- the clinical database solves exactly that problem.
So we're going squarely after the complexity of the trial, the consumption and the analysis of all of those data sets, and then using technology to simplify that rather than this being kind of a one-off services-based project, we want it to be solved with the technology.” — Jun-23 Baird conference
“So we started an application called QualityDocs. That's what you would consider SOP management or standard operating procedure management. There's a procedure in there for cleaning a certain machine that might be very critical, right? To change control around that, the ability to track who knows that. That's where we started.
And then QMS, right? This is a quality management system, things like what's called capitals deviations when you have a quality problems, how you're tracking resolve that. So those, by the way, never have been done together in a common data model by any software vendor because those are very, very different applications. You can't do it unless you have a platform like Vault that both handles content and data, you can't do it. So we did that.
Now then we put training in there. Training is a super important thing. And a company like, for example, AstraZeneca, can you imagine the criticality of training? And now we're adding what's called QC LIMS in there. We're just looking for our first early adopters there, that's maybe the biggest application of them all in the quality area. That's for the workflow around testing — during and after the manufacturing process — quality controls, because when you're making a medicine that goes into the human body, you better believe that's tested rigorously, right, for I think, for very obvious reasons...
So that's why I'm excited about quality, and that's why you saw it in a lower quadrant because the application, for example that we just introduced, just looking for first adopters is actually the biggest application of them all.
So what this leads into? …When we had our customer summit recently and we had the head of quality for actually, I believe that was AstraZeneca, talking on the business side. What they explained is this quality system can improve quality at their company by developing a quality mindset. So it's that strategic.” — 2022 Investor Day
“LIMS is interesting. It is for testing materials, largely pharmaceutical companies, biotechs that make materials in batches. They're not making units, they're making batches, batches of things that then would be packaged. And serious things that are either ingested or intravenous into the human body. So the quality of them is super important for obvious reasons.
LIMS is laboratory information management system, what we call QC or quality control LIMS. That's where you keep the specifications of how you should test this medicine along the way of its life cycle. During its multi-day, could be weeks, process of making this medicine in a batch, what tests should be done? And there's a whole variety of tests, some as simple as what's the color in the pH, to some very sophisticated tests.
Those specifications have to be designed and approved in the system and then data is entered in either through APIs or manual. And that's the quality control process. That's a big part of the quality control process that then is used to see, hey, can we release this medicine in this market to be ingested or injected into humans.
Very important area and we're going to build it inside of our quality Vault, which means it will become unified with our QMS system, our QualityDocs system and our training system. Nobody has ever done anything like that before. And I think it's going to be a real transformation in the quality control processes in life sciences, which will allow people to release their medicines faster, which is a real — it keeps the company much more agile.
So this is very much top of mind in the supply chain area of our customer supply chain and manufacturing. It's a big product. It'll be 1 of our biggest ticket items in the quality suite. It's definitely not an add-on product to QMS or something like that.” — Q4’FY23 earnings
“We like to do what we say we're going to do. In 2015, we announced the goal of $1 billion. In 2015, we said we'd get to $1 billion revenue. We said our goal is to get to $1 billion revenue in 2020. And we reached that a year early. So we reached that in 2019. In 2019 then, we set a new 5-year goal of $3 billion revenue and we're about 1 year ahead of that plan. And we're looking forward to 2030, right? Hopefully, if we can meet our 2025 goal, we'll make a 2030 goal.” — Jan-23 JPM Conference
Dylan Becker
“Got it. Very helpful. So that's a lot, but that's just half of the business, right? So let's talk about the R&D side. There's a number of new products here and to your point, moving from a point solution to a multiproduct strategy here.
Can we walk through each of the components within the R&D suite kind of clinical data management, maybe where each of those fits on that maturity curve and the value of the tightly integrated nature of each of these solutions as well?”
Brent Bowman
“Yes. We'd like to say we planted the seeds for growth. You probably heard that before. But it's a good analogy. We started back in 2012, '13, with our documentation products.
And think about on the clinical side with eTMF and on the quality side with QualityDocs and the regulatory side with Regulatory Submissions. Those products are the largest contributors to our current revenue today. Those are still growing businesses , but they — those seeds were planted over 10 years ago. So really excited about that.
So because we do have, like I said, seeds at different parts of their evolution and their life cycle. The next set of products are really around the operations side. Think about the heartbeat of a clinical operations. So we have software that does that. It's called CTMS.
And I apologize for the alphabet soup, but it is what it is. And then on the quality side, the quality management system, it's running the quality operations.
So those products are really hitting the sweet spot of the growth curve right now and contributing nicely to the growth in '24 and '25 as well. And then what gives us real excitement is our largest applications from a market opportunity, from a TAM perspective, are really just in their infancy. So you talk about clinical data, so our electronic data capture product, and that's under a suite called CDMS, really still early days. We have 6 of the top 20 that have agreed to start all new trials on Veeva.
You don't cut over mid trial. It just doesn't make sense on multiple dimensions. So those 6 have agreed over a period of time. And this could be 3, 4, 5 years to get all the trials on to Veeva, but it's happening. And so it's really exciting.
Safety is another example. We had our second top 20 win in the quarter. Safety is a very risk-adverse area. So we start to see the momentum. Once you get second, third, top 20, that reference selling model really starts kicking in because you can't afford to not be on the best software.
So all this being said, we're creating the operating system for drug development.
We're the only software provider that can actually run the gamut between clinical, regulatory, manufacturing on the quality safety side. And it's all connected on the Vault platform. So our focus is to have best-of-breed app the application level, but also connected. Your alternative is to buy from disparate vendors in all those spaces because there's no other vendor that can go end to end and then you have to find a way -- figure out how to stitch it together. So the value proposition is pretty clear there.”
Dylan Becker
“And sticking with that, too, obviously, right, there are different maturity levels of each of those, but you've seen significant success with the regulatory suite, the quality suite, et cetera, the more established offerings. How do you think about that cross-sell and that attach within that? Serving as maybe like a springboard for what you've talked about in CTMS, clinical data management because you're kind of not flying blind, you have an indication from customers of what they want, and what you can develop?”
Brent Bowman
“No, it's absolutely true. And if you just stick with clinical for a second, and I'll throw out some numbers that kind of gives you a good example of that. So we have 18 of the top 20 on our clinical documentation product, eTMF. We have 11 of the top 20 on our clinical operation products, CTMS. We have 6 of the top 20 in our clinical data product.
The only difference is time and market, but they see the value of that unified clinical suite of products. And then you can take that again outside of that and cut across regulatory safety and quality. So you can start in any one area, but there is that network effect that you see because of that connected nature.” — Jun-23 Blair Conference
Saket Kalia
“Okay. Great. Apologies in advance if these questions have been asked, but I'm going to try it anyway. Peter, maybe first for you, great to see the growth in the EDC customer base. I think you said you added 8, right, in the quarter.
Maybe the question for you is, are there any commonalities that you're seeing across some of those wins, whether it's coming from 1 or 2 specific competitors or maybe a common reasoning that you're seeing those customers choose to switch? Any observations that you would make just as you look at a bigger EDC customer base now?”
Peter Gassner
“Yes, there are some commonalities. I would say the bulk of them are either coming from Medidata, which is the leading by volume in industry solutions, or they're coming from a series of smaller competitors that are targeted for the SMB.
As it relates to Medidata, I would say, they like the EDC solution from Veeva, but they also like the integration with our clinical operations suite. In the EDC solution, why they like our solution better at times, sometimes is — and that will be the primary driver. It's a specific, what I would call meat and potatoes things, for example, when you do a study amendment, you change the design of the study many times with competitive solutions, you have to unload the data, reload the data, the site can't — the clinical research site can't operate during that time with Veeva because of the newer architecture that doesn't happen. We also are able to -- through the use of our tooling, our customers can build their studies, can define their studies dramatically faster, they might do 4 weeks instead of 8 weeks. And then the way that Veeva system works, has less custom programming.
There's custom programming needed in these other solutions. That is expensive, but also [indiscernible] specialized skill, you don't need that, you can define that in Veeva. So these very specific things. And then as to why they would use Veeva instead of maybe smaller SMB solutions, I think, again, they would like to get solutions from 1 partner that fits together. And that's what we do.
A good example of that is we have a great clinical trial management system, and we have a great EDC system. You don't have to use both if you use both or better together. Our ePRO system and our RTSM system, they work fine if you don't have our EDC. They don't require our EDC at all. They're the best stand-alone, and nobody else has done that before, right?
Nobody else that has an EDC product. But if you have all of our products, the integration is even better and it's just easier to get these solutions from an integrated solution from 1 company. So that's what our — what we're seeing. People want a broad clinical partner where all of the applications are excellent, and that's what they can find in Veeva.” — Q2’FY24 earnings
“Medidata is now just competing on price. It's only a matter of time. It's price and habit, so that's it…It's just a matter of time before this Veeva juggernaut just squeezes them out. I'll tell you, Medidata is not putting any more investment into the product.” — Tegus, Former VP of Enterprise CRM Strategy at Veeva, Nov-24
“EDC and the data from EDC flows into the clinical database and the patient-reported outcomes that come from our ePRO product have to end up in the clinical database also in the CDB and it's also related to the randomization that happens, the distribution of the medicines to the right patients obviously impacts the milestones and the trials and the data that you capture. So all of these things are interconnected. So our strategy is, as you — as a customer establishes — it starts in a specific place, happens to be EDC is leading those because it's been in the market longer. Adding the next one becomes even more valuable. And that's what — that's the benefit of these integrated suites of applications. We've seen that play out in many different areas. It's playing out in quality. It's playing out in clinical. It's played out in the commercial side in different parts of our business. So that gives us this unique advantage.” — Jan-24 Needham conference
“I guess to step back, where we have a safety application that's a very complex applications, it's case processing of what's called adverse events. Somebody takes a medicine and they either have a bad reaction or they think they have a bad reaction and then has to be classified and routed and recorded to health authorities, et cetera, around the world. So it's a very complex application. We're really the first company able to do that as a cloud-native application.
That means just like we do everything else, there's one version of the code and all customers run it. So that's a breakthrough. Our customers are used to on-premise applications, they have to have upgrade, lots of issues. Now that this customer is live, it's a top 20, one of the larger top 20 customers, now there's proof that, that works. And I think, I wouldn't say all the way, but mainly customers are thinking, I probably will go with Veeva for safety.
And then the question is when. Is it next year? Is it 3 years from now, 5 years from now, 7 years from now? So that's really the significance of it.
Now we have, of course, the work's never done. I'll never be satisfied with our safety application side. It always has to get better and better and better. But it was a very significant milestone for the industry and for Veeva. And also just the other thing I'd add, the speed that, that implementation happened, I won't quote the exact numbers of days, was probably about half the time that most customers would have expected that it would have taken. So that was also seriously noticed by the industry.” — Q3’FY23 earnings
Anne McCormick
“You highlighted that your safety solutions are approaching a tipping point, and I was wondering if maybe you could provide a little bit more color about what that means. And then could you just remind us of the relative size of this market, what's the unmet need, and where Veeva is differentiated here?”
Peter Gassner
“Yes. So it's more of a feeling when you're approaching a tipping point, it's a little bit different than being at the tipping point. So it's the level of depth of the customer conversations we're having in multiple large customers. Every year, the legacy systems that they're using are aging a little bit. And every year, our cloud-based solutions are getting better and they're getting more optimized and more people are using them.
And it's just kind of like that. You have a feeling that the tipping point is coming. We've also filled out our suite. We have our core safety processing application, which we've had for some time, but now we have Safety Signal and Safety Workbench available. Those are — so those are things they need.
And in terms of what they're looking for is, a, they do want a cloud-based system because they don't — the idea of doing upgrades and taking changes in [indiscernible] stuff is unappealing. A lot of these systems are legacy and aging. So there's, one, just modernization. But the second one is advanced automation. Within the safety system to do some things without manual intervention and then across systems, for example, between clinical data management and the safety system, there's a lot of innovation that we can do because we have both sides of those applications.
So they're looking for automation, not to take the people totally out of the loop, because the people have to be in the loop to know what's going on so that they can care and notice and act appropriately, but to free them up from the redundant activities that they don't need to be doing.” — Q3’FY25 earnings
Ryan MacDonald
“Safety continues to be an interesting area of opportunity. And Brian, you just mentioned it's a sort of a deep area but maybe slower moving than historically. And I know Peter has definitely talked about how complicated that segment with customers are resistant to change, essentially, in safety. Are you starting to see signs of this resistance to change, softening at all? And if so, like as you've had discussions with customers, what do you think the great unlock is to sort of drive a faster rate of change in the safety market?”
Paul Shawah
“Yes. So it is somewhat of a conservative market where they want to get something in place and they don't want to touch it or don't want to change it. But the reality is requirements change, they have to move, they have to evolve it. And doing it with a modern cloud system like safety, like Vault Safety, is a whole lot better. So at some point, it gets to the point where it's too painful to maintain the old thing and it's much better to move at once to transform and become more efficient with Vault.
I think we're at that — we're very close to that tipping point, right? We're starting to see that. We have 3 top 20s. We've executed very well. The industry was watching.
Because these are hard projects to do, right? It's a hard product to build. And it's an important process to get right. And now we're past that point. So you can kind of think of this as like the EDC market playing out.
We had a couple — we had 2 top 20 EDCs that we won. It took us a couple of years to kind of get those live and happy and referenceable and then talking up on a big stage. And we're starting to hit that point in safety. So I feel really good about where we are. And I would say the other thing we've done in safety is not only -- beyond just kind of advancing the core product, is delivering and building out the suite.
So Safety Docs and Signal and Workbench, this is now very unique. It's that unique value proposition of having the suite of products all working together. So yes, we're more and more bullish on where safety is in its life cycle.” — Jan-25 Needham Conference
Craig Hettenbach
“In the R&D segment and understanding the ramping EDC deals are a nice tailwind in the next number of years. Outside of that, any particular areas within R&D that you're most excited about next 1 or 2 years that you expect to really drive growth in that segment?”
Peter Gassner
“In 1 or 2 years. I would say you're right about the EDC, the clinical data management to drive growth. I think the newer products and clinical operations as well. The study training and the Site Connect, I think those are prime to contribute quite well there. Also, a lot of our large — in terms of revenue, are large ramping CTMS deals and QMS deals, right, that are still ramping.” — 2024 Investor Day
“We said leadership means 40% or more market share. Because if you have 40%, you're probably going to be the leader in your -- it means you're going to have somewhere between 40% to 100% over time. That's how that works. If I look at those products, any product we make, I think there's a network effect. So I think we have a good chance to be at 40% or more leader.
And I think I didn't think we -- when we started, I thought it's most likely going to be more of a duopoly type of thing. It turns out there's usually 1 lead dog. And that's how it works. So I don't think there's any difference in any of those products.
There's no structural thing that says, hey, this product area should have 7 providers of equal magnitude. It's not like that. The relentless pursuit of excellence. The markets you can't put enough effort to make 7 great products. The economics don't work out.” — 2022 Investor Day
Craig Hettenbach
“And then just switching gears back to some of the questions on the data business in commercial. Can you touch on just how you're thinking about just from a timeline perspective when you could see some inflection in that business?”
Peter Gassner
“I'll take that one. Real inflection, I do think will be — first, it's going to be customer success wise and product excellence. And that, I think, we'll have a pretty good view about 12 months from now, really. Now that is going to lead the financial significance by a long way. So it will be multiple years from now before — Compass is not going to be a $60 million business, even a $50 million business.
It's not going to be that anytime soon, anytime in the next few years. So that's how long of a path these things are. The leading indicator will be the customer success and the customers — and it would start probably with the very small biotechs who can — who would say, ‘Hey, I run my business, and I don't use any IQVIA data products.’ That's — I just use the Veeva data products and I'm happy with that.
And I'm able to see things that I couldn't see before. That's when you know you have something. So those are the things that we're really looking for every day trying to optimize for, trying to listen for, trying to make changes, so we can arrive at that next year.” — Q2’FY24 earnings
“We're taking a patient-centric approach, patient-centric as opposed to prescription-centric. So the legacy providers, I think in the approach of aggregating and syndicating data from all of the retail pharmacies. Now that's prescription-centric.
We're taking a patient-centric approach, which is looking at diagnoses and procedures and prescriptions, and we're assembling that around a complete view of the patient, which is better for today's more modern medicines. The precision medicines and specialized medicines that we talked about earlier, they need something that's more patient-centric and patient-specific, and that's what we're doing with patient data.
And that's proving out to be very valuable. For our early customers that are using it, they're finding more health care professional targets that they can call on. So this data is more valuable for how they segment and target their customers.” — 2021 Investor Day
“So if you're getting data from three different sources, that they're somewhat overlapping, you have a better chance of getting data quality. And this is one of the reasons why we can project data for medical claims, which is — it might be something that's done in an infusion center.
We can also project for something that you pick up at Walgreens or CVS. Why? Well, because we're sourcing data from multiple points along the line. So the real benefit is more holistic view of the patient, we can project data for over 4,000 brands. You can't do that if you're just projecting based on a few large retailers' data, you can't do that. Because if you look at 20 years ago or 25 when these legacy data products were started, or even when Veeva started in 2007, the biggest brand at the time was Lipitor and you got Lipitor at the drugstore. The biggest brand now is Keytruda and you don't just walk into the drugstore and get that. It doesn't work that way — or HUMIRA, et cetera.
So ours is a modern approach that I think is the only way really to do it well for today's complex therapies. And I do think we'll have lower data cost over time. I do think that, but I don't have to prefer that.” — Q4’FY24 earnings
“Whether we like IQVIA or not from the industry, they have a very strong legacy of data dominance out there. Data being the switch from any data providers to another data providers is higher than a CRM because data is flowing everywhere in the entire organization. It's almost like you are changing the blood of a human being because it's flowing across all the body parts. If you think of CRM, it's just the arms, let's say the R&D is the chest. If CRM is the arm, then you can just replace the arm. On the data side it's going through the body, the legs and everywhere. It's very entrenched on the data side.” — Tegus, Former Dr Product Mgmt at Veeva, Jan-25
“If you look at that $2 trillion, roughly, roughly 1/4 of that. Slightly less than 1/4 of that is medical device of some type or another. So it's an area that we'll focus on. We didn't focus on it so far purely because of customer success. But if you get in too fast, too far, you don't actually go faster, you go slower. So now we're ready and we're really excited about it.” — 2019 Investor Day
“We announced MedTech CRM. Well, we've been working our way in that market and adapting really good. We have a great team working on that, and we decided there we're going to go do it a little different than we've done it in other places. We really went deep into the product for about the last 9 months or so. This is a very deep product MedTech CRM.
So order management specific to MedTech, inventory management, trunk inventory, all that kind of opportunity management, compliant content, right, a lot of stuff in there. And we've developed that deep in the app, and we just signed our first early adopter there. And interestingly enough, they also got at the same time, Link for MedTech. Now that's with a pretty large multibillion-dollar MedTech company, but again, in a small group, right, and we got to grow there. So yes. I think we're planting the seeds.” — 2022 Investor Day
David Hynes
“And maybe one on marketing automation. So you talked about in the past, partnering with Salesforce and Adobe. I think of kind of marketing automation infrastructure as being generally horizontal, right? When you talk about building a verticalized effort in that space that, I guess, displaced some of those relationships? Like what does that entail? How does the product differentiate that positions you to win?”
Paul Shawah
“Yes. You're right. We've had -- we partnered with Salesforce and Adobe. We'll continue to have customers who choose to use them. We're going to create something that's much more integrated into the commercial cloud.
It will be tightly integrated into CRM. That's a big thing, right, because a lot of our — a lot of the solutions that exist today, they're stand-alone. They don't connect in. And they're actually over-architected for what the life sciences industry needs. So we're going to build more precisely what life sciences needs.
I'll give you an example of something that we do in core CRM. In core CRM, you have field teams that use content to talk to doctors, and it's important that, that content is very specific to, let's say, the specialty of that doctor. That's a regulation. You can't show content that's approved for an oncology medicine to, let's say, a general practitioner or pediatrician. That's something that's built into the application.
We would build that kind of thing into the marketing automation system to make sure those rules are respected. That will just be a standard part of the product. It's one example. But — so we'll focus it. We'll make it life sciences specific and it will be tightly integrated and it will give our customers choice.
And the idea here is we want to build out a full and more complete commercial cloud and marketing is a key part of that.” — 2023 Investor Day
“For example, this marketing automation that we're building, I think it could be extra, extra, extra innovative because it will be life sciences specific, it will be regionally aware which is the way you do this stuff in Japan and Germany and the U.S. is quite different in life sciences. And it will be aware of the field, the sales team.
So you could use our marketing automation to inform your sales team of marketing for sure, but let your sales team control some part of marketing or you could use a campaign to control your sales team, all opposite because they're all operating on the same data, the same segment. So it's like having Marketo inside of Salesforce.com and industry specific. That's not been done before.” — Q4’FY24 earnings
“The platform, to understand it, first of all, it's for us. It's for Veeva. It helps us build applications quickly and of high quality and very efficient. That's how we're able to build all those applications you saw, all those big applications. Some companies just struggle just to build one good application.
We have 14 Vault applications now, and that's because of the platform. Fundamentally, much of the code, we can write once in the platform, so we don't have to write it everywhere else. That means you can write it faster. You can focus and have good quality. When you improve your core platform, you're improving all of your applications.
This is a tough thing to build a good cloud platform because you have to use it for developing your own applications and then you have to upgrade and push out the applications. So it's a very interesting thing. All great application companies are going to have one of these. If you show me a company that has acquired lots of different products and/or has built them on different platforms, it's not going to be a good application company. It's not going to have scale.
It's going to break down over time. There's just no way to do it. So we have one, takes a long time to build, but we have one. For our customers, they don't really care how we build the applications. They assume it's going to be great.
They want to use it. But for the customer, it's about the ability to customize their applications, and that's built into the platform, to really change what they need to in that application without breaking the upgrade. They need to integrate with it in a consistent way, consistent set of web services APIs, developer programs, documentation, et cetera. And they need to extend it to create their new tabs, their new objects, to do the things that they need to do. And they get a lot of leverage out of a platform.
If they have multiple applications from Veeva all in the Vault Platform, they start to have common skills on how do I do reporting on these applications? How do I administer security? How do I do an audit? How do I do web services integrations with it? It's the same thing why people like the SAP platform or they like the Workday platform.
What's -- so it's really simple, right? If you don't have one of these great platforms, you don't have a great application company…
When you look in a little deeper, different cloud platforms, all the application platforms, they all have their different specialties. There are areas that they are great at or they are unique at. And here are some unique things to know about Vault.
First off, it's content and data. So what does that mean? Vault is good at handling content like a video. Play a video, stop a video, highlight a certain section of the video, comment on the video. It's good at things like office documents, upload an Office document, render it into an archived PDF, mark it up, diff versions of it. It's good at images. I upload a very complex image, it pulls out the metadata of that image, when was it created, what's its DPI, et cetera. It's good at all that type of content, but it's also good at data, the classic data that you need to track in business applications. What's interesting about that, it's the first platform that's -- that I know of that's ever set out to be great at both. And usually, you'll have something like a Documentum or something like that that's focused on content. You'll have an SAP that's focused on data. We have people that have background from both sides, and that's the people that we used to create Vault, so it can do both.
I would say the process management is a key area. It -- sometimes people call this workflow. I really call it process management. Vault has very complex tools in there to allow you to model a very complex process of workflow, which, for example, you would need to go through when you're getting documents ready to submit a clinical trial or when a pharmaceutical company is trying to approve, for example, a video that they will put on TV. When they do that, there's a lot of steps and a lot of fines if you don't get it right. Is it legally correct? Is it medically correct? Is the compliance with the regulations correct? And who signed off on all this stuff? There's a series of steps. You got to do that accurately and fast. That's process management. And Vault has that built right into it.
Search everything. The fundamental data store, for those of you more on the technical side, is not really a relational database. It's a text search engine. More -- it's more like Google than it is like Oracle. So everything underneath Vault is searchable and superfast. That's why we get the performance and the flexibility that we need. It's also, what I call, a multi-Vault architecture.
When we -- if you would look at the original document that we wrote when we were starting out Vault, it says, "Hey, a large pharmaceutical company should have 15 to 20 Vault applications that Veeva delivers and 15 to 20 that they make on their own. And these are going to come in at different times and be of different maturity." So we have this multi-Vault architecture. So if the regulatory group in pharma gets started first, and they're in, they're live, they're very stable, they're not going to be disrupted by the clinical side coming in, in another Vault. But we have integration between them. In this way, we're quite different from SAP, which has everything in one big mass, and that makes it difficult to coordinate. We have a sort of a federated model to our applications, which we call the multi-Vault architecture.
The security model, and this is somewhat where the real secret sauce is, is probably the most complete that I've seen an application platform, and I've seen a lot of them. We have security right down to the field level on a record. We call it atomic security. And this is really needed in life sciences. We're doing some things that really need a lot of level of security. For example, when you're doing clinical trials and you have a double-blind clinical trial and you need to unblind something. You really can't get that wrong, and you have to do it in an administrative way, not in a coding way.
And then I'll point out what I call real Java. We need to run to the complexity for applications at Veeva because we're doing industry-specific things, and the very last mile really matters. Good enough is not good enough when every application needs to be the leader. So our developers can code when they need to, the business logic in real Java, not some scripting language. And also, we will be exposing that to our customers, too.
So this is a very powerful platform when it needs to be.” — 2017 Investor Day
“Veeva CRM suggestions, partnering with ZS Associates and Oktana to have suggestive actions for the pharmaceutical rep on what to do next across what channels, across what customer, to notice that, oh, a certain patient had a certain medical procedure in Indianapolis, and your prescription sometimes can follow that medical procedure. You should call this doctor tomorrow and — or you should email that doctor this clinical report. This is revenue-generating stuff for our customers, and we are innovating on that.” — 2015 Investor Day
“We're also working on innovation in the safety area and a particular area of innovation is a new product that we're working on called Safety AI. So what does Safety AI do? It uses natural language processing and machine learning to take all of the inbound information that you might get about a safety issue. It could be in a text, in an e-mail, in what's called an E2B case form XML, and it can analyze all that information and consolidate it into cases that are ready for review by a person.
And in talking with some of our early customers, they may have for example 500 people in an offshore location that are responsible for basically combing through this information to create the cases for review. They felt that with this kind of technology, they could drop that number of people from 500 to 200. So it will be a really powerful tool in the Safety arsenal to really expedite case intake, make it more timely, make it much less costly and, as you can imagine, make it much less error-prone as well, by taking at least some of the human intervention out of the equation.” — 2019 Investor Day
Brent Bracelin
“I would love to get your initial thoughts on how Veeva is thinking about incorporating AI and large language models into the business, is there an opportunity to lean in here to either lower cost internally around AI? Or are you thinking about new revenue streams via new products that you could build on kind of these AI advances in large language models?”
Peter Gassner
“Good question about AI. Well, certainly, ChatGPT's kind of taken the world's imagination by storm, and it is a significant type of technology. I won't go too long into that, but it's significant. But to answer your questions, Brent, I don't see any opportunity to really lower internal costs, most of what we do is relationship based in the selling and the marketing, and innovation based, true innovation base and construction in the product area. So I don't see anything there.
As far as for value for our customers, that's something we'll consider over time. We won't rush into it. We have a lot of data in Veeva in the industry cloud overall for the industry. And then we have data — we have our customers' data for each customer. So there's potential that we can do some things to answer some questions about the industry overall, or and/or help the customer answer some questions about their internal operations.
And I do think the large language models are going to be [indiscernible] in the chat type interface. Ask about a question, get a relatively low quality answer that you can kind of move forward from for there. So I think there's a place for it. I don't think it's a revolution, and we'll just see how it goes.” — Q4’FY23 earnings
“We are seeing good uptake of the Direct Data API. And yes, as you mentioned, we recently announced that that's going to be free to all of our customers. And the reason there is we want everybody building on that type of API.
It's just a much better, faster API for many use cases. And we found a way to do it where it was not going to consume as many compute resources as we thought it was. So we're pretty excited about that. We're using it internally, for example, for connecting different parts of our clinical suite, different parts of our safety suite together, and our partners are starting to do it. We have more than 10 customers that are already doing it. Some of them are large customers.
It takes some time because it's a different paradigm for integration. People have been using a hammer for a long time, and now you're giving them a jackhammer and they’ve got to learn how to use it. But we are super enthused. It's a fundamental new type of API where you can get like all of the data out of your Vault super quickly in one S3 file every night and transactionally sound deltas every 15 minutes in one file. Nobody has that type of thing.
And AI is just pumping up the demand for data everywhere, AI and data science. And overall, that's just the trend. As these compute powers get more and more accessible, people want that data in more and more places all the time.
So I'm really enthused about what we're doing for the life sciences industry because many of their core systems are Veeva and now their core systems are going to be enabled with this fundamental new API that's going to allow them to leverage their core data faster than any other industry. So hopefully, we're doing our part to help the life sciences industry grow, and that will be good for Veeva.” — Q4’FY25 earnings
“And then Pulse data…we'll be introducing that product later on this year. That's where we'll make a data set based on the activity in our applications.
So if you look at our CRM application, for example, we do know what's going on in the industry. Our pharmaceutical — how are pharmaceutical companies reaching urologists in the Midwest? Well, we know that. We know that because that's recorded in our system. Now, the trick is to produce that data, produce that set of data in a privacy-safe way globally around the world and use that to enable pharmaceutical companies, for example, to do better segmentation and targeting or engagement planning by knowing what the industry is doing.
And at the same time, we will apply that to clinical. If there was a — if — you can project into the future, a lot of people using the Veeva clinical applications, we might be able to produce a data set that provides visibility into the clinical trial ecosystem.
How many people are on clinical trials? For a clinical trial in this specific disease area, what is the dropout rate? Is Canada trending up and Brazil trending down or not? What's going on? There's — I hope you realize, there's no visibility into that.
There's visibility into prices for stocks that's collected, and you all look at that and you can analyze that. There's not that visibility into what's going on with clinical trials, which are actually experiments on humans and very expensive ones. That's the type of innovation Veeva can bring with the Pulse data.” — Jan-24 JPM conference
“Like I remember, at our customer summit in June, we had one of our large customers presenting their case study and their success with Approved Email. You can't sit in that room as somebody who works at a pharma company and think, oh that big company, my competitor is sending e-mail after they do a call, and that's extending the call, and think, we probably don't have to do that.
That is not what people thought. What people did after that event is they called us and said, we got to do a pilot. Can you help us figure out how to do this? Multichannel is a huge driver, and if you can't be the only pharma company that doesn't do it, and some people call pharma companies dominoes. They're -- No one wants to be first, but they're fast followers.
There's a lot of innovation in this industry. We have plenty of companies who want to be first, but they are fast followers too, and this move to multichannel has absolutely passed the tipping point and this is just how you have to do it in this industry, going forward. And we're the platform that's really enabling it.” — 2015 Investor Day
“Over the years, our subscription gross margin has steadily increased through 2 primary factors: revenue mix and product mix. First, our subscription revenue has historically grown faster than services revenue, which acts as a tailwind to overall gross margin.
Second, on average, Vault products have a materially higher subscription gross margin relative to CRM and some of our other commercial products.” — 2020 Investor Day
“I always had this Mantra that look if our people are not worth the customer paying for them then they're not actually worth anything…So that was a rule. You don't give away professional services. What about if the deal is going to tank? Well then I guess the deal's going to tank, because the one thing we're not going to do is give away professional services…You have to look at yourself if your services are not profitable - gosh why can’t I make services valuable enough? Why do our services cost less than the cost of our people? What am I doing wrong?” — Aug-17 SaaStr conference
“The effectiveness of the Vault platform allows for material innovation and new product introductions while still only spending in the mid-teens in R&D.” — 2020 Investor Day
“Yes, I would just say as we scale, I think we're getting a little more efficient in our processes, in our customer relationships, in our use of our Vault platform, and we're being diligent. That was a concept we call lean teams. Not doing layoffs, but we want lean teams. We want the smallest teams possible with high-performance people that can perform well together.
So this kind of discipline — that's why our margins are increasing. And I think you'll see that from us increasing margins over time.” — Q4’FY24 earnings
“We've always thought about growth and profit together. I think it's something that distinguished Veeva in the early days, and it went out of fashion in the market for a little while there, and maybe it's coming back in fashion right now. I don't really know. But we think about those together and kind of inseparable from each other.
I think sometimes you see companies throw money at an opportunity or throw money at a problem thinking, that's going to accelerate it. That's going to get me there. And I think what we find is that lean teams go faster. And so we always think about taking a disciplined approach to our spending.
And so you're seeing that in some of our margin results right now. Our Q3 margins were at 43.5% non-GAAP, and that's well above the floor of 35% that you mentioned. And we're going to continue to keep that disciplined approach.” — Dec-24 Raymond James conference
Brent Bracelin
“The full year margin guide here, highest we've ever seen, that comes at a time where you're investing in R&D. You have kind of the whole investment focus around a Vault CRM, additional products here. What's driving the guide here above 42% op margin?”
Brian Van Wagener
“Yes, great question. So the main way that we think about margin is investing the right amount in the business. So we've always thought about growth and profitability and don't really view those in conflict with each other. So what you're seeing is that we're always trying to get more efficient, and it's because that efficiency allows us to execute better and faster. We find that lean teams are more agile. They've got better speed.
And so we think there's going to continue to be efficiency and economies of scale. But what we're optimizing for and what you're seeing reflected in the guide is that we're optimizing for speed and execution, not margin. So we're continuing to invest in the business for growth. We're spending about twice on product, what we do on sales and marketing and keeping that focus on product excellence and innovation in our products.” — Q4’FY25 earnings
“So there was an earlier question, how do we decide what to build? And another component of that is we only want to build things where we can get at least half of the market share. In analytics, things like Click and Tableau and Cognos, BusinessObjects, MicroStrategy, all those things. Every one of those companies will tell you that healthcare life science is one of their biggest verticals. Our customers tend to buy them all.
There is no standard in analytics, and if there became a standard, someone would want something different because they view the analysis of data as something that you can't standardize. And so when we look at analytics, it's a huge opportunity. There's a lot of money spent by life sciences companies on advanced analytics tools, but we don't see a path to building something that can get a 50% market share or more. And therefore, we'll let the Tableaus and Clicks of the world fight it out. What we do is we just try to make it easy for our customers to utilize the tool of their choice, which is normally more than one tool.” — 2014 Investor Day
“We live by core values. We have four core values. They used to change quarter by quarter when we first started the company. The first one is ‘do the right thing.’ We didn’t write that down for a long time. But we got to, like, 3-4 thousand employees and we thought, you know what — maybe everyone doesn’t just feel this, let’s write down ‘do the right thing.’ The next one is customer success. Now, when we started the company we had no customers, so customer success wasn’t one of them. The first one when we started the company was ‘speed.’ But now they’re: ‘do the right thing,’ ‘customer success', ‘employee success,’ and ‘speed.’
And it’s been a long time since we changed them, but we review before every big meeting. We review it on the company call every quarter. We review it before every Board meeting. We don’t just list them out the way I just did. We talk about them — and why they’re still important. And when you communicate clearly for many years and you ‘walk the walk,’ then people believe it.
And it’s not ‘cool’ to do something that’s bad for customers at Veeva. It’s not ‘cool’ to do something that hurts another employee. It’s not ‘cool’ to drag your feet, and to wait until the last minute or to slow the team down. That’s just not how we do it. And so the culture of the place is ‘let’s get it done.’ And ‘put the customer first.’
And we’re all busy. No one finishes everything they want to do every day or every week or every quarter. The core values are also up there to say, ‘alright well I’ve got ten things to do, I’ve only got time for one — which is the best for customers, and go there. So it was high-level culture, high-level feeling about the company. But also low-level, I’ve got to make a decision about what I need to do in the next hour. And you can use that as the guide.” — Matt Wallach, Strategy at Scale podcast, 4/8/25
“We have one of our operating principles that says autonomy over alignment, which means we really get paid for innovation, creativity that requires autonomy and sometimes alignment or efficiency is going to suffer because of that. So we're conscious of that. We make that trade-off. We go for autonomy first. And then if we can see a way we can align stuff between our different groups, okay, we do it.
So for example, we don't have a centralized engineering team or a centralized product management team. We have different groups. And inside of Veeva, there are some groups that are start-ups that run completely autonomous. And that's part of — creates our energy. And we want to innovate not only in our technology but in our operating model.” — Jan-23 JPM Conference
“Autonomy is critical when you get to 10,000 people. You have to have autonomous groups. The people we want to attract, they operate better in autonomy. They get a thrill out of they've got the ball. If they drop the ball, it's going right to the floor.
Those are the people we want in the company, that creates innovation so we have to have autonomy, especially for our start-up new business areas that creates the energy and innovation. And we constantly say, ‘We’re okay with some inefficiency.’ That's not — our goal is not an efficiency goal. Our goal is the innovation and customer success goal, so you can't have it both ways.” — 2019 Investor Day
“Speed, that's our third value. Get it done today instead of tomorrow.
Get the thing done. I tell people — and I probably say this 50x a year for sure. It takes 120% effort to win, but it still takes 80% effort to lose. So what we want to do is put in that extra effort and win. Now Veeva has that intensity, and we say in the interview process, it's not the right place for everybody, right?
It has that intensity. We want to get after it every day, every week, every month, every quarter, build something that's significant and deliver value.” — 2014 Investor Day
“This is a little deeper in our operating model than you might want to know, but for each business area, there's twice yearly, very in-depth strategy reviews, where we're looking out 3 or 4 years, and I participate then. There's a set of peers participate in them. And that's where we're really stressing ourselves by area. Are we doing the best we can? Are we getting lazy? Are we getting arrogant? What can we do now to — So yes, this is — I always tell people, David, it's the downside of capitalism. There is no free lunch. There is no resting, right? It's somewhat depressing.
It's an endless game. But if you lose that fire, you'll get — it won't happen. Now also when you're the leader, if you lose that fire, you're not going to be liked and that starts the ball rolling, too. So I want to set a high bar for Veeva and part of that is giving these people the autonomy. We have a virtual CEO of clinical operations and clinical data management — and they know they own that ball. And it's my job to replace them if they don't have the fire, right? That's my job. So I don't want to let our customers down. What if most of the customers are using this clinical software from Veeva, and we start dropping the ball? It's going to slow down the whole industry.
But what if we use our position to gain more market share, to innovate more and then who knows because there's more standardization, we can make some kind of breakthrough that enables a process that really changes the way clinical trials work, wouldn't that be a good thing to do. So I think there's opportunity that can be created on top of a more standardized clinically universe. I've talked to clinical leaders all that time, David, that they don't think the clinical trial process as it is now is really optimized as much as it could be. So I think we're excited. To really answer your question, we're excited about the clinical.
And if there's anybody here working in clinical that's not excited, I need to move them out, really. I mean it's just — I'm sorry to be that blunt, but that's the job.” — 2024 Investor Day
“We say execution matters most. All that other stuff is hoo-ha. I spend 90% of my time executing. What am I going to do today, this week, this month, this year? Write down a plan, execute to a plan. Measure yourself to a plan. Execution matters most because over the long term, any good idea gets copied. But execution is enduring…and it's hard and it's maybe not sexy but it's the thing that will separate you. There’s a downside of capitalism — there's no free lunch. If you want to be great, you have to execute.” — Aug-17 SaaStr conference
“Actually, we bought this company called Veracity Logic about a year ago, a small company but a pretty mature product. We've roughly tripled the team and roughly tripled the revenue in less than a year. And that just shows that — if you show up to your customer with a partnership approach and more products and more products that fit together, you're better off.” — 2022 Investor Day
“We want to then build the best cloud products, and that sounds relatively easy because product — I've been in an enterprise software my whole life. Product is actually the most predictable part of a software company by far because the best product people working for a significant amount of time will produce the best product. Sales, marketing, your competitors, is all are relatively unpredictable. But product is predictable. You just have to assemble the A team.
Now that's where the hard is, how can you assemble the A team. Everybody wants to do that. So you have to work hard at it. You have to know what you're doing. You have to have a company culture and the processes where people can feel good and be productive. You have to — if you want the best artists, you have to have the best artist colony. And that's what we're doing. We work hard on assembling the A team.” — 2016 Investor Day
“So what we've historically talked about is sort of our core revenue, which is around RAVE EDC. And in comparison to the broader platform, our other solutions, including our analytics, and you can think of it as a -- about a 2/3, 1/3 breakdown with 2/3 still coming from core EDC and an about 1/3, from the rest of our solutions. So obviously, the rest of our solutions are growing faster. And over time, we'll see that split become 50-50 and then more.” — Jun-19 Dassault/Medidata M&A call
“So when we began Medidata, we focused on one very specific area, which was electronic data capture. And over the 20-year history of the business, we have, I think, done a very good job of taking market share. And today, we run probably more than half of all electronic trials that are on globally.” — Jun-19 Dassault/Medidata M&A call
“The discussion of a public benefit corporation started probably more than 10 years ago. And the reason it came up was because in Delaware C-corps, the job of the Board of Directors is to maximize shareholder value. That’s the job of the Board. Keep people out of jail, make sure the executives are honest, but — it’s basically maximize shareholder value…And our Board didn’t operate that way. We never talked about the stock price at a Board meeting. We just never did. It was not what we were focused on. And it did not feel like the job of our Board was to maximize shareholder value. It’s not what they did — it’s not how we thought.
The job of the Board was to make sure we were making customers successful, we were doing the right things for the industry, we were creating a great place for people to work. That’s what we focused on in the Board meetings. And so it just felt unnatural and almost dishonest that if you read the articles of incorporation of the company — that’s not the job the board was doing.
And a public benefit corporation, you could choose your stakeholders. And so it took a few years to get ourselves ready for it, to get the Board ready for it. But ultimately we announced that we were going to do it, and then we went on a listening tour — we announced that we were thinking about it. We talked to investors, we talked to customers, partners, employees. And what we heard was that no one actually knew what a public benefit corporation was, so we had to explain it to everyone.
And the way that we positioned it was — listen, we’re in this for the long-term. We care most about customers and employees. And if we do the right thing for customers, and we help the industry, and we create a great place for employees — shareholders will benefit forever. And that we always beleived to be true. But I knew that I was going to retire early. And when we had a discussion with a CEO about buying another Veeva product, and another Veeva product. Sometimes they’d say well listen — we’re spending so many millions of dollars with you guys — how do I know that you’re not going to take advantage of us? And if it was me or Peter we could tell them — listen, you know us, you trust us, we’re good people — we’re not going anywhere. But that can’t last forever, and so we wanted to get it codified forever. To make sure that we would never take advantage of customers.
So part of why you can go all-in with Veeva is because you can trust that we will never take advantage of you. Because as a public benefit corporation — your success, the success of the industry is tied for first with shareholder value. And so we will not do something bad for customers to help shareholders — ever. And we never did. But we could look a CEO in the eye and say I won’t take advantage of you because my Board won’t allow it. And that lasts forever now.” — Matt Wallach, Strategy at Scale podcast, Apr-25
“I always take this analogy. If you see Apple products, I'm a big Apple fan user, so you have a phone and then you have a Watch, you have AirPods, you have MacBook and then all of a sudden if Apple is coming up with the latest AirPods, complete new design, rebranded, do you want to upgrade to the AirPods and their design or do you want to sync AirPods and go to maybe Android pods or something else? It's very similar to that.
When I'm compared to my use, I have a fully integrated system. I can copy in one place, I can paste in other place. Why would I lose that integration and go to another system and get it upgraded even if it is a little bit better?
I would do that if the new product is very exceptional with AI capabilities or whatnot and I'm really losing out, then I would take that extra step and say, "Okay, let me try it out even if I'm not integrating the system but this is really fascinating. Let me go try it out."
It has to make a difference, only then will a partnered customer step out of the integrated hub and go into a brand-new product. Otherwise, I'm disturbing my beautiful ecosystem here. I have all the integrations built out. I have Veeva CRM to PromoMats integration, MedComms integration and PromoMats is talking to RIM and RIM is talking to eTMF.
I have all these connections and ecosystem built out. Why would I just disrupt, take out my CRM and give it to another third party where there is no integration with my other systems? You wouldn't do it unless there is a very big reason, unless the system is too good or it is very cost effective. There should be a big enough reason for you to move away.” — Tegus, 2/7/25, VP, Veeva Services Practice Group at Connexus Solutions
“Here's my personal feeling. Now, it won't match with maybe what you guys will see from the outside. The only way you're going to pull this off is by overpromising a lot of things. The Salesforce guys are promising the sky and the moon, and isn't Veeva so bad and if only you could have AI and data integration, all that?
Wouldn't that be great? The customer will say, "Yeah, damn it, you're right, I'm going to do it." They're going to sign it and declare it and Veeva stock will take a hit from that because "Oh no, this X customer is leaving us." I do not underestimate the difficulty of Salesforce to deliver on something like this because it's not just CRM. Omnichannel is a critical piece of this.
Omnichannel is five other softwares. You need CRM and five other omnichannels and a content management system just to do business today in pharma. Salesforce has to not develop just a CRM, it's got to develop six softwares to make this thing even parity with Veeva. If I already mentioned that Salesforce is not good developing on its own software, imagine not bringing five other softwares into the mix as well.
I'm feeling some people are going to get fired in about two and a half years from now from Salesforce. All those leaders who are overpromising are new to Salesforce too, by the way. Except for Frank Defesche, he was an original person there. All these new people trying to pull off this Manhattan project at Salesforce, overpromising are going to under deliver.
My personal bet is they're going to abandon the project in four years like IQVIA did, but that's very extreme. If you do want to be very good and say, "Hey, well, they'll just give it away for free or a low price," then I would say maybe there's five of the top 50 I can think of that would probably go for a very low cost version of it where they maintain it themselves and make it work for a very low giveaway price. What usually happens is Mark Benioff is just friends with the CEO and says, "Here you go, I'll just give this to you for that," and they agree to this over cocktails at his villa in Hawaii is usually how it goes…
Veeva came in at a very special time when the requirements were very simple. You needed CRM and an offline device like an iPad. Now, we live in a multichannel world. Where doctors in the whole world, 1/4-1/2 of your work is now digital. It takes a long time to get compliant digital omnichannel working. Just to get that alone is a 10-year journey, in my opinion…
I'll just give you a quick example. You have CRM for the storing of the data. You need an offline iPad solution that can work inside the hallways of a hospital. You need compliant email. You need Zoom capabilities that's compliant. You need web portal capabilities. That's what you need just to do the basics of omnichannel today. Those are all examples of five or six softwares that you need.” — Tegus, 11/14/24, CRM Strategy at Veeva